Signalling and putative therapeutic molecules on the regulation of synoviocyte signalling in rheumatoid arthritis
- PMID: 33890482
- PMCID: PMC8077181
- DOI: 10.1302/2046-3758.104.BJR-2020-0331.R1
Signalling and putative therapeutic molecules on the regulation of synoviocyte signalling in rheumatoid arthritis
Abstract
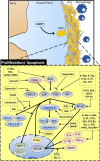
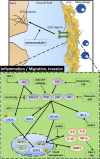
Rheumatoid arthritis (RA) is an autoimmune disease characterized by symmetrical and chronic polyarthritis. Fibroblast-like synoviocytes are mainly involved in joint inflammation and cartilage and bone destruction by inflammatory cytokines and matrix-degrading enzymes in RA. Approaches that induce various cellular growth alterations of synoviocytes are considered as potential strategies for treating RA. However, since synoviocytes play a critical role in RA, the mechanism and hyperplastic modulation of synoviocytes and their motility need to be addressed. In this review, we focus on the alteration of synoviocyte signalling and cell fate provided by signalling proteins, various antioxidant molecules, enzymes, compounds, clinical candidates, to understand the pathology of the synoviocytes, and finally to achieve developed therapeutic strategies of RA. Cite this article: Bone Joint Res 2021;10(4):285-297.
Keywords: Hyperplasia; Inflammation; Joint destruction; Rheumatoid arthritis; Synoviocytes.
Figures
Similar articles
-
Cyanidin prevents the hyperproliferative potential of fibroblast-like synoviocytes and disease progression via targeting IL-17A cytokine signalling in rheumatoid arthritis.Toxicol Appl Pharmacol. 2020 Mar 15;391:114917. doi: 10.1016/j.taap.2020.114917. Epub 2020 Feb 7. Toxicol Appl Pharmacol. 2020. PMID: 32044269
-
Leonurine attenuates fibroblast-like synoviocyte-mediated synovial inflammation and joint destruction in rheumatoid arthritis.Rheumatology (Oxford). 2017 Aug 1;56(8):1417-1427. doi: 10.1093/rheumatology/kex142. Rheumatology (Oxford). 2017. PMID: 28431044
-
Histone demethylase JMJD3 regulates fibroblast-like synoviocyte-mediated proliferation and joint destruction in rheumatoid arthritis.FASEB J. 2018 Jul;32(7):4031-4042. doi: 10.1096/fj.201701483R. Epub 2018 Feb 26. FASEB J. 2018. PMID: 29481307
-
Fibroblast-like synoviocytes-dependent effector molecules as a critical mediator for rheumatoid arthritis: Current status and future directions.Int Rev Immunol. 2017 Jan 2;36(1):20-30. doi: 10.1080/08830185.2016.1269175. Epub 2017 Jan 19. Int Rev Immunol. 2017. PMID: 28102734 Review.
-
Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling.Cell Signal. 2013 Oct;25(10):2069-78. doi: 10.1016/j.cellsig.2013.04.002. Epub 2013 Apr 17. Cell Signal. 2013. PMID: 23602936 Review.
Cited by
-
CCL2 promotes osteogenesis by facilitating macrophage migration during acute inflammation.Front Cell Dev Biol. 2023 Jun 30;11:1213641. doi: 10.3389/fcell.2023.1213641. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37457301 Free PMC article.
-
Immunomodulatory role of T helper cells in rheumatoid arthritis : a comprehensive research review.Bone Joint Res. 2022 Jul;11(7):426-438. doi: 10.1302/2046-3758.117.BJR-2021-0594.R1. Bone Joint Res. 2022. PMID: 35775145 Free PMC article.
-
Sex differences of NF-κB-targeted therapy for mitigating osteoporosis associated with chronic inflammation of bone.Bone Joint Res. 2024 Jan 10;13(1):28-39. doi: 10.1302/2046-3758.131.BJR-2023-0040.R3. Bone Joint Res. 2024. PMID: 38194999 Free PMC article.
-
Multiple-Factors-Induced Rheumatoid Arthritis Synoviocyte Activation Is Attenuated by the α2-Adrenergic Receptor Agonist Dexmedetomidine.Int J Mol Sci. 2023 Jun 28;24(13):10756. doi: 10.3390/ijms241310756. Int J Mol Sci. 2023. PMID: 37445932 Free PMC article.
-
Mucin 1 aggravates synovitis and joint damage of rheumatoid arthritis by regulating inflammation and aggression of fibroblast-like synoviocytes.Bone Joint Res. 2022 Sep;11(9):639-651. doi: 10.1302/2046-3758.119.BJR-2021-0398.R2. Bone Joint Res. 2022. PMID: 36048147 Free PMC article.
References
-
- Singh JA, Saag KG, Bridges SL, et al. . 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(1):1–26. - PubMed
-
- Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. - PubMed
-
- Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. The Lancet. 2007;370(9602):1861–1874. - PubMed
-
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. The Lancet. 2016;388(10055):2023–2038. - PubMed

