Identification of regulated proteins by epigallocatechin gallate treatment in an ischemic cerebral cortex animal model: a proteomics approach
- PMID: 33883340
- PMCID: PMC8267205
- DOI: 10.1292/jvms.21-0089
Identification of regulated proteins by epigallocatechin gallate treatment in an ischemic cerebral cortex animal model: a proteomics approach
Abstract
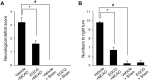
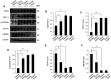
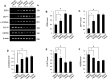
Ischemic stroke is a fatal disease that has long-term disability. It induces excessive oxidative stress generation and cellular metabolic disorders, result in tissue damage. Epigallocatechin gallate (EGCG) is a naturally derived flavonoid with strong antioxidant property. We previously reported the neuroprotective effect of EGCG in ischemic stroke. The defensive mechanisms of stroke are very diverse and complex. This study investigated specific proteins that are regulated by EGCG treatment in the ischemic brain damage. Middle cerebral artery occlusion (MCAO) was performed to induce focal cerebral ischemia. EGCG (50 mg/kg) or vehicle was intraperitoneally administered just prior to MCAO. MCAO induced severe neurological deficits and disorders. EGCG treatment alleviated these neurological disorder and damage. Cerebral cortex was used for this study. Two-dimensional gel electrophoresis and mass spectrometry were performed to detect the proteins altered by EGCG. We identified various proteins that were changed between vehicle- and EGCG-treated animals. Among these proteins, isocitrate dehydrogenase, dynamin-like protein 1, and γ-enolase were decreased in vehicle-treated animals, while EGCG treatment prevented these decreases. However, pyridoxal-5'-phosphate phosphatase and 60 kDa heat shock protein were increased in vehicle-treated animals with MCAO injury. EGCG treatment attenuated these increases. The changes in these proteins were confirmed by Western blot and reverse transcription-PCR analyses. These proteins were associated with cellular metabolism and neuronal regeneration. Thus, these findings can suggest that EGCG performs a defensive mechanism in ischemic damage by regulating specific proteins related to energy metabolism and neuronal protection.
Keywords: epigallocatechin gallate; proteomics; stroke.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Epigallocatechin gallate improves neuronal damage in animal model of ischemic stroke and glutamate-exposed neurons via modulation of hippocalcin expression.PLoS One. 2024 Mar 1;19(3):e0299042. doi: 10.1371/journal.pone.0299042. eCollection 2024. PLoS One. 2024. PMID: 38427657 Free PMC article.
-
Epigallocatechin gallate alleviates neuronal cell damage against focal cerebral ischemia in rats.J Vet Med Sci. 2020 May 20;82(5):639-645. doi: 10.1292/jvms.19-0703. Epub 2020 Mar 30. J Vet Med Sci. 2020. PMID: 32224555 Free PMC article.
-
Epigallocatechin Gallate Alleviates Down-Regulation of Thioredoxin in Ischemic Brain Damage and Glutamate-Exposed Neuron.Neurochem Res. 2021 Nov;46(11):3035-3049. doi: 10.1007/s11064-021-03403-0. Epub 2021 Jul 29. Neurochem Res. 2021. PMID: 34327632
-
Identification of Proteins Differentially Expressed by Quercetin Treatment in a Middle Cerebral Artery Occlusion Model: A Proteomics Approach.Neurochem Res. 2018 Aug;43(8):1608-1623. doi: 10.1007/s11064-018-2576-x. Epub 2018 Jun 20. Neurochem Res. 2018. PMID: 29926355
-
Advances in the Anti-Atherosclerotic Mechanisms of Epigallocatechin Gallate.Nutrients. 2024 Jun 28;16(13):2074. doi: 10.3390/nu16132074. Nutrients. 2024. PMID: 38999821 Free PMC article. Review.
Cited by
-
Epigallocatechin gallate improves neuronal damage in animal model of ischemic stroke and glutamate-exposed neurons via modulation of hippocalcin expression.PLoS One. 2024 Mar 1;19(3):e0299042. doi: 10.1371/journal.pone.0299042. eCollection 2024. PLoS One. 2024. PMID: 38427657 Free PMC article.
-
Regulation of DAPK1 by Natural Products: An Important Target in Treatment of Stroke.Neurochem Res. 2022 Aug;47(8):2142-2157. doi: 10.1007/s11064-022-03628-7. Epub 2022 Jun 8. Neurochem Res. 2022. PMID: 35674928 Review.
-
Proteomic advance of ischemic stroke: preclinical, clinical, and intervention.Metab Brain Dis. 2023 Dec;38(8):2521-2546. doi: 10.1007/s11011-023-01262-y. Epub 2023 Jul 13. Metab Brain Dis. 2023. PMID: 37440002 Review.
References
-
- Barsoum M. J., Yuan H., Gerencser A. A., Liot G., Kushnareva Y., Gräber S., Kovacs I., Lee W. D., Waggoner J., Cui J., White A. D., Bossy B., Martinou J. C., Youle R. J., Lipton S. A., Ellisman M. H., Perkins G. A., Bossy-Wetzel E.2006. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 25: 3900–3911. doi: 10.1038/sj.emboj.7601253 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

