The Morphogenetic Protein CotE Positions Exosporium Proteins CotY and ExsY during Sporulation of Bacillus cereus
- PMID: 33883264
- PMCID: PMC8546674
- DOI: 10.1128/mSphere.00007-21
The Morphogenetic Protein CotE Positions Exosporium Proteins CotY and ExsY during Sporulation of Bacillus cereus
Abstract
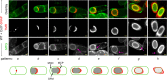
The exosporium is the outermost spore layer of some Bacillus and Clostridium species and related organisms. It mediates the interactions of spores with their environment, modulates spore adhesion and germination, and has been implicated in pathogenesis. In Bacillus cereus, the exosporium consists of a crystalline basal layer, formed mainly by the two cysteine-rich proteins CotY and ExsY, surrounded by a hairy nap composed of glycoproteins. The morphogenetic protein CotE is necessary for the integrity of the B. cereus exosporium, but how CotE directs exosporium assembly remains unknown. Here, we used super-resolution fluorescence microscopy to follow the localization of SNAP-tagged CotE, CotY, and ExsY during B. cereus sporulation and evidenced the interdependencies among these proteins. Complexes of CotE, CotY, and ExsY are present at all sporulation stages, and the three proteins follow similar localization patterns during endospore formation that are reminiscent of the localization pattern of Bacillus subtilis CotE. We show that B. cereus CotE guides the formation of one cap at both forespore poles by positioning CotY and then guides forespore encasement by ExsY, thereby promoting exosporium elongation. By these two actions, CotE ensures the formation of a complete exosporium. Importantly, we demonstrate that the assembly of the exosporium is not a unidirectional process, as previously proposed, but occurs through the formation of two caps, as observed during B. subtilis coat morphogenesis, suggesting that a general principle governs the assembly of the spore surface layers of BacillaceaeIMPORTANCE Spores of Bacillaceae are enveloped in an outermost glycoprotein layer. In the B. cereus group, encompassing the Bacillus anthracis and B. cereus pathogens, this layer is easily recognizable by a characteristic balloon-like appearance and separation from the underlying coat by an interspace. In spite of its importance for the environmental interactions of spores, including those with host cells, the mechanism of assembly of the exosporium is poorly understood. We used super-resolution fluorescence microscopy to directly visualize the formation of the exosporium during the sporulation of B. cereus, and we studied the localization and interdependencies of proteins essential for exosporium morphogenesis. We discovered that these proteins form a morphogenetic scaffold before a complete exosporium or coat is detectable. We describe how the different proteins localize to the scaffold and how they subsequently assemble around the spore, and we present a model for the assembly of the exosporium.
Keywords: SR-SIM; endospores; exosporium; morphogenetic proteins; spore.
Copyright © 2021 Lablaine et al.
Figures
Similar articles
-
ExsY, CotY, and CotE Effects on Bacillus anthracis Outer Spore Layer Architecture.J Bacteriol. 2022 Nov 15;204(11):e0029122. doi: 10.1128/jb.00291-22. Epub 2022 Oct 4. J Bacteriol. 2022. PMID: 36194010 Free PMC article.
-
ExsY and CotY are required for the correct assembly of the exosporium and spore coat of Bacillus cereus.J Bacteriol. 2006 Nov;188(22):7905-13. doi: 10.1128/JB.00997-06. Epub 2006 Sep 15. J Bacteriol. 2006. PMID: 16980471 Free PMC article.
-
Sporulation Temperature Reveals a Requirement for CotE in the Assembly of both the Coat and Exosporium Layers of Bacillus cereus Spores.Appl Environ Microbiol. 2015 Oct 23;82(1):232-43. doi: 10.1128/AEM.02626-15. Print 2016 Jan 1. Appl Environ Microbiol. 2015. PMID: 26497467 Free PMC article.
-
Structure, assembly, and function of the spore surface layers.Annu Rev Microbiol. 2007;61:555-88. doi: 10.1146/annurev.micro.61.080706.093224. Annu Rev Microbiol. 2007. PMID: 18035610 Review.
-
The Bacillus anthracis Exosporium: What's the Big "Hairy" Deal?Microbiol Spectr. 2015 Oct;3(5). doi: 10.1128/microbiolspec.TBS-0021-2015. Microbiol Spectr. 2015. PMID: 26542035 Review.
Cited by
-
ExsY, CotY, and CotE Effects on Bacillus anthracis Outer Spore Layer Architecture.J Bacteriol. 2022 Nov 15;204(11):e0029122. doi: 10.1128/jb.00291-22. Epub 2022 Oct 4. J Bacteriol. 2022. PMID: 36194010 Free PMC article.
-
Changes in the Spore Proteome of Bacillus cereus in Response to Introduction of Plasmids.Microorganisms. 2022 Aug 24;10(9):1695. doi: 10.3390/microorganisms10091695. Microorganisms. 2022. PMID: 36144297 Free PMC article.
-
Comparing divisome organization between vegetative and sporulating Bacillus subtilis at the nanoscale using DNA-PAINT.Sci Adv. 2024 Jan 12;10(2):eadk5847. doi: 10.1126/sciadv.adk5847. Epub 2024 Jan 10. Sci Adv. 2024. PMID: 38198550 Free PMC article.
-
Roles and Organization of BxpB (ExsFA) and ExsFB in the Exosporium Outer Basal Layer of Bacillus anthracis.J Bacteriol. 2022 Dec 20;204(12):e0029022. doi: 10.1128/jb.00290-22. Epub 2022 Nov 17. J Bacteriol. 2022. PMID: 36394311 Free PMC article.
-
Localization of the CotY and ExsY proteins to the exosporium basal layer of Bacillus anthracis.Microbiologyopen. 2022 Oct;11(5):e1327. doi: 10.1002/mbo3.1327. Microbiologyopen. 2022. PMID: 36314748 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
