Comprehensive analysis and identification of drought-responsive candidate NAC genes in three semi-arid tropics (SAT) legume crops
- PMID: 33882825
- PMCID: PMC8059324
- DOI: 10.1186/s12864-021-07602-5
Comprehensive analysis and identification of drought-responsive candidate NAC genes in three semi-arid tropics (SAT) legume crops
Abstract
Background: Chickpea, pigeonpea, and groundnut are the primary legume crops of semi-arid tropics (SAT) and their global productivity is severely affected by drought stress. The plant-specific NAC (NAM - no apical meristem, ATAF - Arabidopsis transcription activation factor, and CUC - cup-shaped cotyledon) transcription factor family is known to be involved in majority of abiotic stresses, especially in the drought stress tolerance mechanism. Despite the knowledge available regarding NAC function, not much information is available on NAC genes in SAT legume crops.
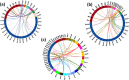
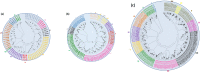
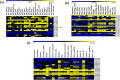
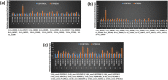
Results: In this study, genome-wide NAC proteins - 72, 96, and 166 have been identified from the genomes of chickpea, pigeonpea, and groundnut, respectively, and later grouped into 10 clusters in chickpea and pigeonpea, while 12 clusters in groundnut. Phylogeny with well-known stress-responsive NACs in Arabidopsis thaliana, Oryza sativa (rice), Medicago truncatula, and Glycine max (soybean) enabled prediction of putative stress-responsive NACs in chickpea (22), pigeonpea (31), and groundnut (33). Transcriptome data revealed putative stress-responsive NACs at various developmental stages that showed differential expression patterns in the different tissues studied. Quantitative real-time PCR (qRT-PCR) was performed to validate the expression patterns of selected stress-responsive, Ca_NAC (Cicer arietinum - 14), Cc_NAC (Cajanus cajan - 15), and Ah_NAC (Arachis hypogaea - 14) genes using drought-stressed and well-watered root tissues from two contrasting drought-responsive genotypes of each of the three legumes. Based on expression analysis, Ca_06899, Ca_18090, Ca_22941, Ca_04337, Ca_04069, Ca_04233, Ca_12660, Ca_16379, Ca_16946, and Ca_21186; Cc_26125, Cc_43030, Cc_43785, Cc_43786, Cc_22429, and Cc_22430; Ah_ann1.G1V3KR.2, Ah_ann1.MI72XM.2, Ah_ann1.V0X4SV.1, Ah_ann1.FU1JML.2, and Ah_ann1.8AKD3R.1 were identified as potential drought stress-responsive candidate genes.
Conclusion: As NAC genes are known to play role in several physiological and biological activities, a more comprehensive study on genome-wide identification and expression analyses of the NAC proteins have been carried out in chickpea, pigeonpea and groundnut. We have identified a total of 21 potential drought-responsive NAC genes in these legumes. These genes displayed correlation between gene expression, transcriptional regulation, and better tolerance against drought. The identified candidate genes, after validation, may serve as a useful resource for molecular breeding for drought tolerance in the SAT legume crops.
Keywords: Chickpea; Drought tolerance; Groundnut; Legumes; NACs; Phylogenetics; Pigeonpea; cis-acting regulatory elements (CARE).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Advances in genetics and molecular breeding of three legume crops of semi-arid tropics using next-generation sequencing and high-throughput genotyping technologies.J Biosci. 2012 Nov;37(5):811-20. doi: 10.1007/s12038-012-9228-0. J Biosci. 2012. PMID: 23107917 Review.
-
Genome-wide dissection of AP2/ERF and HSP90 gene families in five legumes and expression profiles in chickpea and pigeonpea.Plant Biotechnol J. 2016 Jul;14(7):1563-77. doi: 10.1111/pbi.12520. Epub 2016 Jan 23. Plant Biotechnol J. 2016. PMID: 26800652 Free PMC article.
-
A comprehensive resource of drought- and salinity- responsive ESTs for gene discovery and marker development in chickpea (Cicer arietinum L.).BMC Genomics. 2009 Nov 15;10:523. doi: 10.1186/1471-2164-10-523. BMC Genomics. 2009. PMID: 19912666 Free PMC article.
-
Exciting journey of 10 years from genomes to fields and markets: Some success stories of genomics-assisted breeding in chickpea, pigeonpea and groundnut.Plant Sci. 2016 Jan;242:98-107. doi: 10.1016/j.plantsci.2015.09.009. Epub 2015 Sep 10. Plant Sci. 2016. PMID: 26566828 Review.
-
NAC transcription factor genes: genome-wide identification, phylogenetic, motif and cis-regulatory element analysis in pigeonpea (Cajanus cajan (L.) Millsp.).Mol Biol Rep. 2014 Dec;41(12):7763-73. doi: 10.1007/s11033-014-3669-5. Epub 2014 Aug 10. Mol Biol Rep. 2014. PMID: 25108674
Cited by
-
Geographically Disperse, Culturable Seed-Associated Microbiota in Forage Plants of Alfalfa (Medicago sativa L.) and Pitch Clover (Bituminaria bituminosa L.): Characterization of Beneficial Inherited Strains as Plant Stress-Tolerance Enhancers.Biology (Basel). 2022 Dec 16;11(12):1838. doi: 10.3390/biology11121838. Biology (Basel). 2022. PMID: 36552347 Free PMC article.
-
Genome-wide identification, evolution, and expression analysis of the NAC gene family in chestnut (Castanea mollissima).Front Genet. 2024 Jan 25;15:1337578. doi: 10.3389/fgene.2024.1337578. eCollection 2024. Front Genet. 2024. PMID: 38333622 Free PMC article.
-
Genome-Wide Identification of the NAC Gene Family in Zanthoxylum bungeanum and Their Transcriptional Responses to Drought Stress.Int J Mol Sci. 2022 Apr 26;23(9):4769. doi: 10.3390/ijms23094769. Int J Mol Sci. 2022. PMID: 35563160 Free PMC article.
-
Genome-wide analyses of the mung bean NAC gene family reveals orthologs, co-expression networking and expression profiling under abiotic and biotic stresses.BMC Plant Biol. 2022 Jul 15;22(1):343. doi: 10.1186/s12870-022-03716-4. BMC Plant Biol. 2022. PMID: 35836131 Free PMC article.
-
Drought Tolerance in Plants: Physiological and Molecular Responses.Plants (Basel). 2024 Oct 23;13(21):2962. doi: 10.3390/plants13212962. Plants (Basel). 2024. PMID: 39519881 Free PMC article. Review.
References
-
- Lewis G, Schrire B, Mackinder B, Lock M. Legumes of the world. Royal Botanic Gardens: Kew; 2005.
-
- FAOSTAT. Production crops data. 2018. http://www.fao.org/faostat/en/#data/QC. Accessed 15 Oct 2020.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

