Different Neurogenic Potential in the Subnuclei of the Postnatal Rat Cochlear Nucleus
- PMID: 33880121
- PMCID: PMC8046557
- DOI: 10.1155/2021/8871308
Different Neurogenic Potential in the Subnuclei of the Postnatal Rat Cochlear Nucleus
Abstract
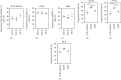
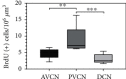
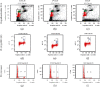
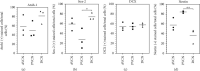
In patients suffering from hearing loss, the reduced or absent neural input induces morphological changes in the cochlear nucleus (CN). Neural stem cells have recently been identified in this first auditory relay. Afferent nerve signals and their impact on the immanent neural stem and progenitor cells already impinge upon the survival of early postnatal cells within the CN. This auditory brainstem nucleus consists of three different subnuclei: the anteroventral cochlear nucleus (AVCN), the posteroventral cochlear nucleus (PVCN), and the dorsal cochlear nucleus (DCN). Since these subdivisions differ ontogenetically and physiologically, the question arose whether regional differences exist in the neurogenic niche. CN from postnatal day nine Sprague-Dawley rats were microscopically dissected into their subnuclei and cultivated in vitro as free-floating cell cultures and as whole-mount organ cultures. In addition to cell quantifications, immunocytological and immunohistological studies of the propagated cells and organ preparations were performed. The PVCN part showed the highest mitotic potential, while the AVCN and DCN had comparable activity. Specific stem cell markers and the ability to differentiate into cells of the neural lineage were detected in all three compartments. The present study shows that in all subnuclei of rat CN, there is a postnatal neural stem cell niche, which, however, differs significantly in its potential. The results can be explained by the origin from different regions in the rhombic lip, the species, and the various analysis techniques applied. In conclusion, the presented results provide further insight into the neurogenic potential of the CN, which may prove beneficial for the development of new regenerative strategies for hearing loss.
Copyright © 2021 Johannes Voelker et al.
Conflict of interest statement
We declare that we have no financial interests that might be relevant to the submitted work.
Figures





Similar articles
-
Trauma-specific insults to the cochlear nucleus in the rat.J Neurosci Res. 2012 Oct;90(10):1924-31. doi: 10.1002/jnr.23093. Epub 2012 Jun 20. J Neurosci Res. 2012. PMID: 22715005
-
Quantitative changes in calretinin immunostaining in the cochlear nuclei after unilateral cochlear removal in young ferrets.J Comp Neurol. 2005 Mar 21;483(4):458-75. doi: 10.1002/cne.20437. J Comp Neurol. 2005. PMID: 15700274 Free PMC article.
-
Neonatal deafness results in degraded topographic specificity of auditory nerve projections to the cochlear nucleus in cats.J Comp Neurol. 2006 Jul 1;497(1):13-31. doi: 10.1002/cne.20968. J Comp Neurol. 2006. PMID: 16680765 Free PMC article.
-
Effects of brain-derived neurotrophic factor (BDNF) on the cochlear nucleus in cats deafened as neonates.Hear Res. 2016 Dec;342:134-143. doi: 10.1016/j.heares.2016.10.011. Epub 2016 Oct 20. Hear Res. 2016. PMID: 27773647 Free PMC article.
-
Intrinsic connections within and between cochlear nucleus subdivisions in cat.J Comp Neurol. 1988 Dec 8;278(2):209-25. doi: 10.1002/cne.902780205. J Comp Neurol. 1988. PMID: 3230161
Cited by
-
Spontaneous Calcium Oscillations through Differentiation: A Calcium Imaging Analysis of Rat Cochlear Nucleus Neural Stem Cells.Cells. 2021 Oct 19;10(10):2802. doi: 10.3390/cells10102802. Cells. 2021. PMID: 34685782 Free PMC article.
References
-
- Moore J. K., Niparko J. K., Miller M. R., Linthicum F. H. Effect of profound hearing loss on a central auditory nucleus. The American Journal of Otology. 1994;15(5):588–595. - PubMed
-
- Kandel E., Schwartz J., Jessell T., Siegelbaum S., Hudspeth A. J. Principles of Neural Science. McGraw-Hill Professional: Fifth Edition; 2012.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

