Caveolar peroxynitrite formation impairs endothelial TRPV4 channels and elevates pulmonary arterial pressure in pulmonary hypertension
- PMID: 33879616
- PMCID: PMC8092599
- DOI: 10.1073/pnas.2023130118
Caveolar peroxynitrite formation impairs endothelial TRPV4 channels and elevates pulmonary arterial pressure in pulmonary hypertension
Abstract
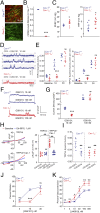
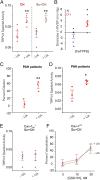
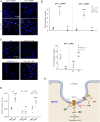
Recent studies have focused on the contribution of capillary endothelial TRPV4 channels to pulmonary pathologies, including lung edema and lung injury. However, in pulmonary hypertension (PH), small pulmonary arteries are the focus of the pathology, and endothelial TRPV4 channels in this crucial anatomy remain unexplored in PH. Here, we provide evidence that TRPV4 channels in endothelial cell caveolae maintain a low pulmonary arterial pressure under normal conditions. Moreover, the activity of caveolar TRPV4 channels is impaired in pulmonary arteries from mouse models of PH and PH patients. In PH, up-regulation of iNOS and NOX1 enzymes at endothelial cell caveolae results in the formation of the oxidant molecule peroxynitrite. Peroxynitrite, in turn, targets the structural protein caveolin-1 to reduce the activity of TRPV4 channels. These results suggest that endothelial caveolin-1-TRPV4 channel signaling lowers pulmonary arterial pressure, and impairment of endothelial caveolin-1-TRPV4 channel signaling contributes to elevated pulmonary arterial pressure in PH. Thus, inhibiting NOX1 or iNOS activity, or lowering endothelial peroxynitrite levels, may represent strategies for restoring vasodilation and pulmonary arterial pressure in PH.
Keywords: TRP channel; caveolin; endothelium; peroxynitrite; pulmonary hypertension.
Conflict of interest statement
The authors declare no competing interest.
Figures





Similar articles
-
Local Peroxynitrite Impairs Endothelial Transient Receptor Potential Vanilloid 4 Channels and Elevates Blood Pressure in Obesity.Circulation. 2020 Apr 21;141(16):1318-1333. doi: 10.1161/CIRCULATIONAHA.119.043385. Epub 2020 Feb 3. Circulation. 2020. PMID: 32008372 Free PMC article.
-
Endothelial pannexin 1-TRPV4 channel signaling lowers pulmonary arterial pressure in mice.Elife. 2021 Sep 7;10:e67777. doi: 10.7554/eLife.67777. Elife. 2021. PMID: 34490843 Free PMC article.
-
Nitric Oxide-Dependent Feedback Loop Regulates Transient Receptor Potential Vanilloid 4 (TRPV4) Channel Cooperativity and Endothelial Function in Small Pulmonary Arteries.J Am Heart Assoc. 2017 Dec 23;6(12):e007157. doi: 10.1161/JAHA.117.007157. J Am Heart Assoc. 2017. PMID: 29275372 Free PMC article.
-
Emerging roles of calcium-activated K channels and TRPV4 channels in lung oedema and pulmonary circulatory collapse.Acta Physiol (Oxf). 2017 Jan;219(1):176-187. doi: 10.1111/apha.12768. Epub 2016 Sep 16. Acta Physiol (Oxf). 2017. PMID: 27497091 Review.
-
Endothelial TRPV4 channels and vasodilator reactivity.Curr Top Membr. 2020;85:89-117. doi: 10.1016/bs.ctm.2020.01.007. Epub 2020 Feb 12. Curr Top Membr. 2020. PMID: 32402646 Free PMC article. Review.
Cited by
-
Role of TRP ion channels in cerebral circulation and neurovascular communication.Neurosci Lett. 2021 Nov 20;765:136258. doi: 10.1016/j.neulet.2021.136258. Epub 2021 Sep 22. Neurosci Lett. 2021. PMID: 34560190 Free PMC article. Review.
-
Mechanosensitive channels in lung disease.Front Physiol. 2023 Nov 15;14:1302631. doi: 10.3389/fphys.2023.1302631. eCollection 2023. Front Physiol. 2023. PMID: 38033335 Free PMC article. Review.
-
Transient receptor potential vanilloid 4 channel inhibition attenuates lung ischemia-reperfusion injury in a porcine lung transplant model.J Thorac Cardiovasc Surg. 2024 Oct;168(4):e121-e132. doi: 10.1016/j.jtcvs.2024.03.001. Epub 2024 Apr 27. J Thorac Cardiovasc Surg. 2024. PMID: 38678474
-
Oxidative Stress and Cerebral Vascular Tone: The Role of Reactive Oxygen and Nitrogen Species.Int J Mol Sci. 2024 Mar 5;25(5):3007. doi: 10.3390/ijms25053007. Int J Mol Sci. 2024. PMID: 38474253 Free PMC article. Review.
-
Single-Cell RNA-Seq Reveals Coronary Heterogeneity and Identifies CD133+TRPV4high Endothelial Subpopulation in Regulating Flow-Induced Vascular Tone in Mice.Arterioscler Thromb Vasc Biol. 2024 Mar;44(3):653-665. doi: 10.1161/ATVBAHA.123.319516. Epub 2024 Jan 25. Arterioscler Thromb Vasc Biol. 2024. PMID: 38269590 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

