Advanced genetic engineering to achieve in vivo targeting of adenovirus utilizing camelid single domain antibody
- PMID: 33872627
- PMCID: PMC10292108
- DOI: 10.1016/j.jconrel.2021.04.009
Advanced genetic engineering to achieve in vivo targeting of adenovirus utilizing camelid single domain antibody
Abstract
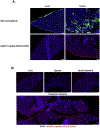
For the developing field of gene therapy the successful address of the basic requirement effective gene delivery has remained a critical barrier. In this regard, the "Holy Grail" vector envisioned by the field's pioneers embodied the ability to achieve efficient and specific in vivo gene delivery. Functional linkage of antibody selectivity with viral vector efficiency represented a logical strategy but has been elusive. Here we have addressed this key issue by developing the technical means to pair antibody-based targeting with adenoviral-mediated gene transfer. Our novel method allows efficient and specific gene delivery. Importantly, our studies validated the achievement of this key vectorology mandate in the context of in vivo gene delivery. Vectors capable of effective in vivo delivery embody the potential to dramatically expand the range of successful gene therapy cures.
Keywords: Adenoviral vectors (Ad); CD276 [B7-H3]; Camelid single domain antibody (sdAb); Gene delivery; Human epithelial ovarian cancer cell (SKOV3.ip1); Ovarian Cancer (OvCa) xenograft mouse model.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
No potential conflicts of interest were disclosed.
Figures

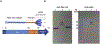

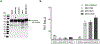
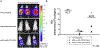
Similar articles
-
Targeting Tumor Neoangiogenesis via Targeted Adenoviral Vector to Achieve Effective Cancer Gene Therapy for Disseminated Neoplastic Disease.Mol Cancer Ther. 2020 Mar;19(3):966-971. doi: 10.1158/1535-7163.MCT-19-0768. Epub 2020 Jan 6. Mol Cancer Ther. 2020. PMID: 31907220 Free PMC article.
-
Localized adenovirus gene delivery using antiviral IgG complexation.Gene Ther. 2001 May;8(9):659-67. doi: 10.1038/sj.gt.3301452. Gene Ther. 2001. PMID: 11406760
-
Modification of an adenoviral vector with biologically selected peptides: a novel strategy for gene delivery to cells of choice.Hum Gene Ther. 1999 Nov 1;10(16):2615-26. doi: 10.1089/10430349950016654. Hum Gene Ther. 1999. PMID: 10566889
-
Adenovirus as an integrating vector.Curr Gene Ther. 2002 May;2(2):135-44. doi: 10.2174/1566523024605591. Curr Gene Ther. 2002. PMID: 12109211 Review.
-
Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy.Curr Gene Ther. 2002 May;2(2):111-33. doi: 10.2174/1566523024605618. Curr Gene Ther. 2002. PMID: 12109210 Review.
Cited by
-
Redirect Tropism of Fowl Adenovirus 4 Vector by Modifying Fiber2 with Variable Domain of Heavy-Chain Antibody.Genes (Basel). 2024 Apr 8;15(4):467. doi: 10.3390/genes15040467. Genes (Basel). 2024. PMID: 38674401 Free PMC article.
-
Recent Advancement in Breast Cancer Research: Insights from Model Organisms-Mouse Models to Zebrafish.Cancers (Basel). 2023 May 29;15(11):2961. doi: 10.3390/cancers15112961. Cancers (Basel). 2023. PMID: 37296923 Free PMC article. Review.
-
Discovery of nanobodies: a comprehensive review of their applications and potential over the past five years.J Nanobiotechnology. 2024 Oct 26;22(1):661. doi: 10.1186/s12951-024-02900-y. J Nanobiotechnology. 2024. PMID: 39455963 Free PMC article. Review.
-
A Novel Piggyback Strategy for mRNA Delivery Exploiting Adenovirus Entry Biology.Viruses. 2022 Sep 30;14(10):2169. doi: 10.3390/v14102169. Viruses. 2022. PMID: 36298724 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

