Rethinking the biology of metastatic melanoma: a holistic approach
- PMID: 33870460
- PMCID: PMC8213587
- DOI: 10.1007/s10555-021-09960-8
Rethinking the biology of metastatic melanoma: a holistic approach
Abstract
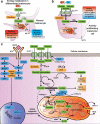
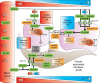
Over the past decades, melanoma-related mortality has remained nearly stable. The main reason is treatment failure of metastatic disease and the inherently linked knowledge gap regarding metastasis formation. In order to elicit invasion, melanoma cells manipulate the tumor microenvironment, gain motility, and adhere to the extracellular matrix and cancer-associated fibroblasts. Melanoma cells thereby express different cell adhesion molecules like laminins, integrins, N-cadherin, and others. Epithelial-mesenchymal transition (EMT) is physiological during embryologic development, but reactivated during malignancy. Despite not being truly epithelial, neural crest-derived malignancies like melanoma share similar biological programs that enable tumorigenesis, invasion, and metastasis. This complex phenomenon is termed phenotype switching and is intertwined with oncometabolism as well as dormancy escape. Additionally, it has been shown that primary melanoma shed exosomes that create a favorable premetastatic niche in the microenvironment of secondary organs and lymph nodes. Although the growing body of literature describes the aforementioned concepts separately, an integrative holistic approach is missing. Using melanoma as a tumor model, this review will shed light on these complex biological principles in an attempt to clarify the mechanistic metastatic pathways that dictate tumor and patient fate.
Keywords: Dormancy; Epithelial-mesenchymal transition; Melanoma; Metastasis; Phenotype switching; Warburg effect.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Melanoma cell-derived exosomes promote epithelial-mesenchymal transition in primary melanocytes through paracrine/autocrine signaling in the tumor microenvironment.Cancer Lett. 2016 Jul 1;376(2):318-27. doi: 10.1016/j.canlet.2016.03.050. Epub 2016 Apr 7. Cancer Lett. 2016. PMID: 27063098 Free PMC article.
-
Wnt-signaling enhances neural crest migration of melanoma cells and induces an invasive phenotype.Mol Cancer. 2018 Feb 17;17(1):59. doi: 10.1186/s12943-018-0773-5. Mol Cancer. 2018. PMID: 29454361 Free PMC article.
-
Epithelial-to-mesenchymal-like transition events in melanoma.FEBS J. 2022 Mar;289(5):1352-1368. doi: 10.1111/febs.16021. Epub 2021 May 30. FEBS J. 2022. PMID: 33999497 Review.
-
FGFR3 promotes the growth and malignancy of melanoma by influencing EMT and the phosphorylation of ERK, AKT, and EGFR.BMC Cancer. 2019 Oct 16;19(1):963. doi: 10.1186/s12885-019-6161-8. BMC Cancer. 2019. PMID: 31619201 Free PMC article.
-
The role of exosomes in metastasis and progression of melanoma.Cancer Treat Rev. 2020 Apr;85:101975. doi: 10.1016/j.ctrv.2020.101975. Epub 2020 Jan 22. Cancer Treat Rev. 2020. PMID: 32050108 Review.
Cited by
-
Cyclooxygenase-2 (COX-2) Expression in Equine Melanocytic Tumors.Vet Sci. 2024 Feb 7;11(2):77. doi: 10.3390/vetsci11020077. Vet Sci. 2024. PMID: 38393095 Free PMC article.
-
Pigmentation Levels Affect Melanoma Responses to Coriolus versicolor Extract and Play a Crucial Role in Melanoma-Mononuclear Cell Crosstalk.Int J Mol Sci. 2021 May 27;22(11):5735. doi: 10.3390/ijms22115735. Int J Mol Sci. 2021. PMID: 34072104 Free PMC article.
-
Capsaicin and Cold exposure promote EMT-mediated premetastatic niche formation to facilitate colorectal cancer metastasis.J Cancer. 2024 Jan 1;15(2):356-369. doi: 10.7150/jca.83985. eCollection 2024. J Cancer. 2024. Retraction in: J Cancer. 2024 Apr 9;15(10):3151. doi: 10.7150/jca.96595 PMID: 38169517 Free PMC article. Retracted.
-
The Genetic Basis of Dormancy and Awakening in Cutaneous Metastatic Melanoma.Cancers (Basel). 2022 Apr 23;14(9):2104. doi: 10.3390/cancers14092104. Cancers (Basel). 2022. PMID: 35565234 Free PMC article. Review.
-
Vessel co-option and angiotropic extravascular migratory metastasis: a continuum of tumour growth and spread?Br J Cancer. 2022 Apr;126(7):973-980. doi: 10.1038/s41416-021-01686-2. Epub 2022 Jan 5. Br J Cancer. 2022. PMID: 34987186 Free PMC article. Review.
References
-
- Yun S, Vincelette ND, Green MR, Wahner Hendrickson AE, Abraham I. Targeting immune checkpoints in unresectable metastatic cutaneous melanoma: A systematic review and meta-analysis of anti-CTLA-4 and anti-PD-1 agents trials. Cancer Medicine. 2016;5(7):1481–1491. doi: 10.1002/cam4.732. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

