Validation of a Quick Flow Cytometry-Based Assay for Acute Infection Based on CD64 and CD169 Expression. New Tools for Early Diagnosis in COVID-19 Pandemic
- PMID: 33869256
- PMCID: PMC8044950
- DOI: 10.3389/fmed.2021.655785
Validation of a Quick Flow Cytometry-Based Assay for Acute Infection Based on CD64 and CD169 Expression. New Tools for Early Diagnosis in COVID-19 Pandemic
Abstract
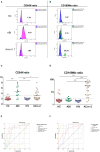
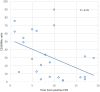
Objectives: Several parameters aid in deciphering between viral and bacterial infections; however, new tools should be investigated in order to reduce the time to results and proceed with an early target-therapy. Validation of a biomarker study, including CD64 and CD169 expression, was conducted. Material and Methods: Patients with active SARS-CoV-2 infection (ACov-2), bacterial infection (ABI), healthy controls, and antiretroviral-controlled chronic HIV infection were assessed. Whole blood was stained and, after lysing no-wash protocol, acquired by flow cytometry. The median fluorescence intensity (MFI) of CD64 and CD169 was measured in granulocytes, monocytes, and lymphocytes. The CD64 MFI ratio granulocytes to lymphocytes (CD64N) and CD169 MFI ratio monocytes to lymphocytes (CD169Mo) were evaluated as biomarkers of acute bacterial and viral infection, respectively. Results: A CD64N ratio higher than 3.3 identified patients with ABI with 83.3 and 85.9% sensitivity and specificity, with an area under the curve (AUC) of 83.5%. In contrast, other analytic or hematological parameters used in the clinic had lower AUC compared with the CD64N ratio. Moreover, a CD169Mo ratio higher than 3.3 was able to identify ACov-2 with 91.7 and 89.8 sensitivity and specificity, with the highest AUC (92.0%). Conclusion: This work confirms the previous data of CD64N and CD169Mo ratios in an independent cohort, including controlled chronic viral HIV infection patients as biomarkers of acute bacterial and viral infections, respectively. Such an approach would benefit from quick pathogen identification for a direct-therapy with a clear application in different Health Care Units, especially during this COVID pandemic.
Keywords: CD169; CD64; SARS-CoV-2; biomarkers; flow cytometry; validation.
Copyright © 2021 Comins-Boo, Gutiérrez-Larrañaga, Roa-Bautista, Guiral Foz, Renuncio García, González López, Irure Ventura, Fariñas-Álvarez, San Segundo and López Hoyos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
CD64 expression on neutrophils as a potential biomarker for bacterial infection in ascitic fluid of cirrhotic patients.Infect Dis (Lond). 2023 Sep;55(9):646-652. doi: 10.1080/23744235.2023.2223294. Epub 2023 Jun 13. Infect Dis (Lond). 2023. PMID: 37310691
-
Kinetics of CD169, HLA-DR, and CD64 expression as predictive biomarkers of SARS-CoV2 outcome.J Anesth Analg Crit Care. 2023 Mar 27;3(1):6. doi: 10.1186/s44158-023-00090-x. J Anesth Analg Crit Care. 2023. PMID: 37386613 Free PMC article.
-
Accuracy of CD64 expression on neutrophils and monocytes in bacterial infection diagnosis at pediatric intensive care admission.Eur J Clin Microbiol Infect Dis. 2019 Jun;38(6):1079-1085. doi: 10.1007/s10096-019-03497-z. Epub 2019 Feb 2. Eur J Clin Microbiol Infect Dis. 2019. PMID: 30712229
-
Myeloid CD169/Siglec1: An immunoregulatory biomarker in viral disease.Front Med (Lausanne). 2022 Sep 23;9:979373. doi: 10.3389/fmed.2022.979373. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36213653 Free PMC article. Review.
-
Neutrophil CD64 expression as a biomarker in the early diagnosis of bacterial infection: a meta-analysis.Int J Infect Dis. 2013 Jan;17(1):e12-23. doi: 10.1016/j.ijid.2012.07.017. Epub 2012 Aug 31. Int J Infect Dis. 2013. PMID: 22940278 Review.
Cited by
-
A New Easy-to-Perform Flow Cytometry Assay for Determining Bacterial- and Viral-Infection-Induced Polymorphonuclear Neutrophil and Monocyte Membrane Marker Modulation in Febrile Patients.Int J Mol Sci. 2024 Oct 29;25(21):11632. doi: 10.3390/ijms252111632. Int J Mol Sci. 2024. PMID: 39519183 Free PMC article.
-
Diagnostic Accuracy of Liquid Biomarkers in Airway Diseases: Toward Point-of-Care Applications.Front Med (Lausanne). 2022 Jun 6;9:855250. doi: 10.3389/fmed.2022.855250. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35733871 Free PMC article. Review.
-
Monocyte HLADR and Immune Dysregulation Index as Biomarkers for COVID-19 Severity and Mortality.Indian J Clin Biochem. 2023 Apr;38(2):204-211. doi: 10.1007/s12291-022-01087-z. Epub 2022 Oct 6. Indian J Clin Biochem. 2023. PMID: 36246016 Free PMC article.
-
Persistence of circulating CD169+monocytes and HLA-DR downregulation underline the immune response impairment in PASC individuals: the potential contribution of different COVID-19 pandemic waves.Curr Res Microb Sci. 2023 Dec 12;6:100215. doi: 10.1016/j.crmicr.2023.100215. eCollection 2024. Curr Res Microb Sci. 2023. PMID: 38187999 Free PMC article.
-
High CD169 Monocyte/Lymphocyte Ratio Reflects Immunophenotype Disruption and Oxygen Need in COVID-19 Patients.Pathogens. 2021 Dec 18;10(12):1639. doi: 10.3390/pathogens10121639. Pathogens. 2021. PMID: 34959594 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

