Translational Animal Models Provide Insight Into Mesenchymal Stromal Cell (MSC) Secretome Therapy
- PMID: 33869217
- PMCID: PMC8044970
- DOI: 10.3389/fcell.2021.654885
Translational Animal Models Provide Insight Into Mesenchymal Stromal Cell (MSC) Secretome Therapy
Abstract
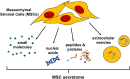
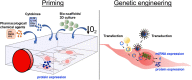
The therapeutic potential of the mesenchymal stromal cell (MSC) secretome, consisting of all molecules secreted by MSCs, is intensively studied. MSCs can be readily isolated, expanded, and manipulated in culture, and few people argue with the ethics of their collection. Despite promising pre-clinical studies, most MSC secretome-based therapies have not been implemented in human medicine, in part because the complexity of bioactive factors secreted by MSCs is not completely understood. In addition, the MSC secretome is variable, influenced by individual donor, tissue source of origin, culture conditions, and passage. An increased understanding of the factors that make up the secretome and the ability to manipulate MSCs to consistently secrete factors of biologic importance will improve MSC therapy. To aid in this goal, we can draw from the wealth of information available on secreted factors from MSC isolated from veterinary species. These translational animal models will inspire efforts to move human MSC secretome therapy from bench to bedside.
Keywords: human; mesenchymal stromal cells; secretome; stem cells; translational models; veterinary.
Copyright © 2021 Harman, Marx and Van de Walle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
An overview of the current advances in the treatment of inflammatory diseases using mesenchymal stromal cell secretome.Immunopharmacol Immunotoxicol. 2023 Dec;45(4):497-507. doi: 10.1080/08923973.2023.2180388. Epub 2023 Feb 28. Immunopharmacol Immunotoxicol. 2023. PMID: 36786742 Review.
-
Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo.Acta Biomater. 2019 Nov;99:247-257. doi: 10.1016/j.actbio.2019.09.022. Epub 2019 Sep 17. Acta Biomater. 2019. PMID: 31539656 Free PMC article.
-
Mesenchymal stromal cell spheroids in sulfated alginate enhance muscle regeneration.Acta Biomater. 2023 Jan 1;155:271-281. doi: 10.1016/j.actbio.2022.10.054. Epub 2022 Nov 1. Acta Biomater. 2023. PMID: 36328130
-
Uncovering the secretome of mesenchymal stromal cells exposed to healthy, traumatic, and degenerative intervertebral discs: a proteomic analysis.Stem Cell Res Ther. 2021 Jan 7;12(1):11. doi: 10.1186/s13287-020-02062-2. Stem Cell Res Ther. 2021. PMID: 33413584 Free PMC article.
-
The secretome of mesenchymal stem cells and oxidative stress: challenges and opportunities in cell-free regenerative medicine.Mol Biol Rep. 2021 Jul;48(7):5607-5619. doi: 10.1007/s11033-021-06360-7. Epub 2021 Jun 30. Mol Biol Rep. 2021. PMID: 34191238 Review.
Cited by
-
ADSCs stimulated by VEGF-C alleviate intestinal inflammation via dual mechanisms of enhancing lymphatic drainage by a VEGF-C/VEGFR-3-dependent mechanism and inhibiting the NF-κB pathway by the secretome.Stem Cell Res Ther. 2022 Sep 5;13(1):448. doi: 10.1186/s13287-022-03132-3. Stem Cell Res Ther. 2022. PMID: 36064450 Free PMC article.
-
Acoustofluidic Interfaces for the Mechanobiological Secretome of MSCs.Nat Commun. 2023 Nov 22;14(1):7639. doi: 10.1038/s41467-023-43239-6. Nat Commun. 2023. PMID: 37993431 Free PMC article.
-
Dynamic Changes of the Bone Marrow Niche: Mesenchymal Stromal Cells and Their Progeny During Aging and Leukemia.Front Cell Dev Biol. 2021 Aug 10;9:714716. doi: 10.3389/fcell.2021.714716. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34447754 Free PMC article. Review.
-
Immortalized mammosphere-derived epithelial cells retain a bioactive secretome with antimicrobial, regenerative, and immunomodulatory properties.Stem Cell Res Ther. 2024 Nov 14;15(1):429. doi: 10.1186/s13287-024-04019-1. Stem Cell Res Ther. 2024. PMID: 39543714 Free PMC article.
-
Mesenchymal stromal cells modulate infection and inflammation in the uterus and mammary gland.BMC Vet Res. 2023 Mar 30;19(1):64. doi: 10.1186/s12917-023-03616-1. BMC Vet Res. 2023. PMID: 36997964 Free PMC article.
References
-
- Ahn J. O., Lee H. W., Seo K., Kang S. K., Ra J. C., Youn H. Y. (2013). Anti-tumor effect of adipose tissue derived-mesenchymal stem cells expressing interferon-β and treatment with cisplatin in a xenograft mouse model for canine melanoma. PLoS One 8:e74897. 10.1371/journal.pone.0074897 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

