Transcriptional and Immunologic Correlates of Response to Pandemic Influenza Vaccine in Aviremic, HIV-Infected Children
- PMID: 33868267
- PMCID: PMC8044856
- DOI: 10.3389/fimmu.2021.639358
Transcriptional and Immunologic Correlates of Response to Pandemic Influenza Vaccine in Aviremic, HIV-Infected Children
Abstract
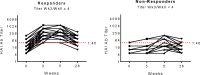
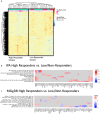
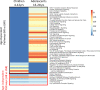
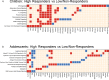
People living with HIV (PWH) often exhibit poor responses to influenza vaccination despite effective combination anti-retroviral (ART) mediated viral suppression. There exists a paucity of data in identifying immune correlates of influenza vaccine response in context of HIV infection that would be useful in improving its efficacy in PWH, especially in younger individuals. Transcriptomic data were obtained by microarray from whole blood isolated from aviremic pediatric and adolescent HIV-infected individuals (4-25 yrs) given two doses of Novartis/H1N1 09 vaccine during the pandemic H1N1 influenza outbreak. Supervised clustering and gene set enrichment identified contrasts between individuals exhibiting high and low antibody responses to vaccination. High responders exhibited hemagglutination inhibition antibody titers >1:40 post-first dose and 4-fold increase over baseline. Baseline molecular profiles indicated increased gene expression in metabolic stress pathways in low responders compared to high responders. Inflammation-related and interferon-inducible gene expression pathways were higher in low responders 3 wks post-vaccination. The broad age range and developmental stage of participants in this study prompted additional analysis by age group (e.g. <13yrs and ≥13yrs). This analysis revealed differential enrichment of gene pathways before and after vaccination in the two age groups. Notably, CXCR5, a homing marker expressed on T follicular helper (Tfh) cells, was enriched in high responders (>13yrs) following vaccination which was accompanied by peripheral Tfh expansion. Our results comprise a valuable resource of immune correlates of vaccine response to pandemic influenza in HIV infected children that may be used to identify favorable targets for improved vaccine design in different age groups.
Keywords: HIV; influenza; microarray; pandemic; pediatric; systems vaccinology; vaccine.
Copyright © 2021 de Armas, George, Filali-Mouhim, Steel, Parmigiani, Cunningham, Weinberg, Trautmann, Sekaly, Cameron and Pahwa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Responses to pandemic ASO3-adjuvanted A/California/07/09 H1N1 influenza vaccine in human immunodeficiency virus-infected individuals.BMC Immunol. 2012 Aug 31;13:49. doi: 10.1186/1471-2172-13-49. BMC Immunol. 2012. PMID: 22937824 Free PMC article.
-
Induction of IL21 in Peripheral T Follicular Helper Cells Is an Indicator of Influenza Vaccine Response in a Previously Vaccinated HIV-Infected Pediatric Cohort.J Immunol. 2017 Mar 1;198(5):1995-2005. doi: 10.4049/jimmunol.1601425. Epub 2017 Jan 27. J Immunol. 2017. PMID: 28130496 Free PMC article.
-
Response to 2009 pandemic and seasonal influenza vaccines co-administered to HIV-infected and HIV-uninfected former drug users living in a rehabilitation community in Italy.Vaccine. 2011 Nov 15;29(49):9209-13. doi: 10.1016/j.vaccine.2011.09.103. Epub 2011 Oct 3. Vaccine. 2011. PMID: 21974995
-
Key points in evaluating immunogenicity of pandemic influenza vaccines: A lesson from immunogenicity studies of influenza A(H1N1)pdm09 vaccine.Vaccine. 2017 Sep 18;35(39):5303-5308. doi: 10.1016/j.vaccine.2017.07.092. Epub 2017 Aug 4. Vaccine. 2017. PMID: 28784284 Review.
-
B cell function and influenza vaccine responses in healthy aging and disease.Curr Opin Immunol. 2014 Aug;29:112-8. doi: 10.1016/j.coi.2014.05.008. Epub 2014 Jun 14. Curr Opin Immunol. 2014. PMID: 24934648 Free PMC article. Review.
Cited by
-
Nutritional Supplementation to Increase Influenza Vaccine Response in Children Living With HIV: A Pilot Clinical Trial.Front Pediatr. 2022 Jul 19;10:919753. doi: 10.3389/fped.2022.919753. eCollection 2022. Front Pediatr. 2022. PMID: 35928688 Free PMC article.
-
State-of-the-Science of human papillomavirus vaccination in women with human immunodeficiency Virus: Summary of a scientific workshop.Prev Med Rep. 2023 Jul 19;35:102331. doi: 10.1016/j.pmedr.2023.102331. eCollection 2023 Oct. Prev Med Rep. 2023. PMID: 37576844 Free PMC article. Review.
-
The role of cell-mediated immunity against influenza and its implications for vaccine evaluation.Front Immunol. 2022 Aug 16;13:959379. doi: 10.3389/fimmu.2022.959379. eCollection 2022. Front Immunol. 2022. PMID: 36052083 Free PMC article. Review.
-
Deciphering Immune Responses to Immunization via Transcriptional Analysis: A Narrative Review of the Current Evidence towards Personalized Vaccination Strategies.Int J Mol Sci. 2024 Jun 28;25(13):7095. doi: 10.3390/ijms25137095. Int J Mol Sci. 2024. PMID: 39000206 Free PMC article. Review.
References
-
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2019-20 Influenza Season. MMWR Recomm Rep (2019) 68(3):1–21. 10.15585/mmwr.rr6803a1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

