Effect of maternal praziquantel treatment for Schistosoma japonicum infection on the offspring susceptibility and immunologic response to infection at age six, a cohort study
- PMID: 33861768
- PMCID: PMC8081342
- DOI: 10.1371/journal.pntd.0009328
Effect of maternal praziquantel treatment for Schistosoma japonicum infection on the offspring susceptibility and immunologic response to infection at age six, a cohort study
Abstract
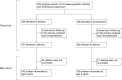
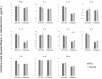
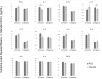
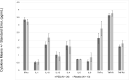
In areas endemic to schistosomiasis, fetal exposure to schistosome antigens prime the offspring before potential natural infection. Praziquantel (PZQ) treatment for Schistosoma japonicum infection in pregnant women has been demonstrated to be safe and effective. Our objectives were to evaluate whether maternal PZQ treatment modifies the process of in utero sensitization to schistosome antigens potentially impacting later risk of infection, as well as immune response to S. japonicum. We enrolled 295 children at age six, born to mothers with S. japonicum infection who participated in a randomized control trial of PZQ versus placebo given at 12-16 weeks gestation in Leyte, The Philippines. At enrollment, we assessed and treated current S. japonicum infection and measured serum cytokines. During a follow-up visit four weeks later, we assessed peripheral blood mononuclear cell (PBMC) cytokine production in response to soluble worm antigen preparation (SWAP) or soluble egg antigen (SEA). Associations between maternal treatment group and the child's S. japonicum infection status and immunologic responses were determined using multivariate linear regression analysis. PZQ treatment during pregnancy did not impact the prevalence (P = 0.12) or intensity (P = 0.59) of natural S. japonicum infection among children at age six. Among children with infection at enrollment (12.5%) there were no significant serum cytokine concentration differences between maternal treatment groups. Among children with infection at enrollment, IL-1 production by PBMCs stimulated with SEA was higher (P = 0.03) in the maternal PZQ group compared to placebo. Among children without infection, PBMCs stimulated with SEA produced greater IL-12 (P = 0.03) and with SWAP produced less IL-4 (P = 0.01) in the maternal PZQ group compared to placebo. Several cytokines produced by PBMCs in response to SWAP and SEA were significantly higher in children with S. japonicum infection irrespective of maternal treatment: IL-4, IL-5, IL-10, and IL-13. We report that maternal PZQ treatment for S. japonicum shifted the PBMC immune response to a more inflammatory signature but had no impact on their offspring's likelihood of infection or serum cytokines at age six, further supporting the safe use of PZQ in pregnant women. Trial Registration: ClinicalTrials.gov NCT00486863.
Conflict of interest statement
The authors have declared that no competing interests exist. Author Remigio M. Olveda was unable to confirm their authorship contributions. On their behalf, the corresponding author has reported their contributions to the best of their knowledge
Figures
Similar articles
-
Effect of praziquantel treatment of Schistosoma mansoni during pregnancy on immune responses to schistosome antigens among the offspring: results of a randomised, placebo-controlled trial.BMC Infect Dis. 2011 Sep 2;11:234. doi: 10.1186/1471-2334-11-234. BMC Infect Dis. 2011. PMID: 21888656 Free PMC article. Clinical Trial.
-
Maternal, placental and cord blood cytokines and the risk of adverse birth outcomes among pregnant women infected with Schistosoma japonicum in the Philippines.PLoS Negl Trop Dis. 2019 Jun 12;13(6):e0007371. doi: 10.1371/journal.pntd.0007371. eCollection 2019 Jun. PLoS Negl Trop Dis. 2019. PMID: 31188820 Free PMC article. Clinical Trial.
-
Praziquantel promotes protection against Schistosoma japonicum infection in mice.Acta Trop. 2023 May;241:106874. doi: 10.1016/j.actatropica.2023.106874. Epub 2023 Mar 1. Acta Trop. 2023. PMID: 36863502
-
Praziquantel treatment after Schistosoma japonicum infection maintains hepatic insulin sensitivity and improves glucose metabolism in mice.Parasit Vectors. 2017 Oct 2;10(1):453. doi: 10.1186/s13071-017-2400-5. Parasit Vectors. 2017. PMID: 28969688 Free PMC article.
-
Th2 cytokines are associated with persistent hepatic fibrosis in human Schistosoma japonicum infection.J Infect Dis. 2007 Jan 15;195(2):288-95. doi: 10.1086/510313. Epub 2006 Dec 13. J Infect Dis. 2007. PMID: 17191174
Cited by
-
How correlations between treatment access and surveillance inclusion impact neglected tropical disease monitoring and evaluation-A simulated study.PLoS Negl Trop Dis. 2023 Sep 6;17(9):e0011582. doi: 10.1371/journal.pntd.0011582. eCollection 2023 Sep. PLoS Negl Trop Dis. 2023. PMID: 37672518 Free PMC article.
References
-
- GBD Disease Injury Incidence Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. Epub 2018/11/30. 10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

