Sustained Release GLP-1 Agonist PT320 Delays Disease Progression in a Mouse Model of Parkinson's Disease
- PMID: 33860208
- PMCID: PMC8033754
- DOI: 10.1021/acsptsci.1c00013
Sustained Release GLP-1 Agonist PT320 Delays Disease Progression in a Mouse Model of Parkinson's Disease
Abstract
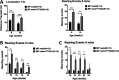
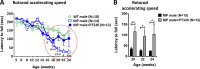
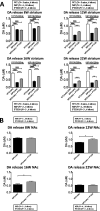
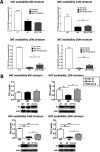
GLP-1 agonists have become increasingly interesting as a new Parkinson's disease (PD) clinical treatment strategy. Additional preclinical studies are important to validate this approach and define the disease stage when they are most effective. We hence characterized the efficacy of PT320, a sustained release formulation of the long acting GLP-1 agonist, exenatide, in a progressive PD (MitoPark) mouse model. A clinically translatable biweekly PT320 dose was administered starting at 5 weeks of age and longitudinally evaluated to 24 weeks, and multiple behavioral/cellular parameters were measured. PT320 significantly improved spontaneous locomotor activity and rearing in MitoPark PD mice. "Motivated" behavior also improved, evaluated by accelerating rotarod performance. Behavioral improvement was correlated with enhanced cellular and molecular indices of dopamine (DA) midbrain function. Fast scan cyclic voltammetry demonstrated protection of striatal and nucleus accumbens DA release and reuptake in PT320 treated MitoPark mice. Positron emission tomography showed protection of striatal DA fibers and tyrosine hydroxylase protein expression was augmented by PT320 administration. Early PT320 treatment may hence provide an important neuroprotective therapeutic strategy in PD.
© 2021 The Authors. Published byAmerican Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): J.J. and H.C. are employees of Peptron Inc. D.S.C. is a scientific advisor to Peptron, Inc. The Intramural Research Program of the National Institute on Aging, NIH, and Peptron, Inc., have a Cooperative Research and Development Agreement to develop Ex-4 as a treatment strategy for neurodegenerative disorders for which NIA and Peptron, Inc., hold patent rights via the work of H.C. and N.G. L.O. is a former co-owner of a company owning commercial rights to the MitoPark mouse.
Figures

Similar articles
-
PT320, a Sustained-Release GLP-1 Receptor Agonist, Ameliorates L-DOPA-Induced Dyskinesia in a Mouse Model of Parkinson's Disease.Int J Mol Sci. 2023 Feb 28;24(5):4687. doi: 10.3390/ijms24054687. Int J Mol Sci. 2023. PMID: 36902115 Free PMC article.
-
Attenuating mitochondrial dysfunction and morphological disruption with PT320 delays dopamine degeneration in MitoPark mice.J Biomed Sci. 2024 Apr 17;31(1):38. doi: 10.1186/s12929-024-01025-6. J Biomed Sci. 2024. PMID: 38627765 Free PMC article.
-
Release parameters during progressive degeneration of dopamine neurons in a mouse model reveal earlier impairment of spontaneous than forced behaviors.J Neurochem. 2019 Jul;150(1):56-73. doi: 10.1111/jnc.14702. Epub 2019 May 9. J Neurochem. 2019. PMID: 30933310 Free PMC article.
-
Voluntary exercise delays progressive deterioration of markers of metabolism and behavior in a mouse model of Parkinson's disease.Brain Res. 2019 Oct 1;1720:146301. doi: 10.1016/j.brainres.2019.146301. Epub 2019 Jun 18. Brain Res. 2019. PMID: 31226324 Free PMC article. Review.
-
Imaging in Parkinson's disease: the role of monoamines in behavior.Biol Psychiatry. 2006 May 15;59(10):908-18. doi: 10.1016/j.biopsych.2005.12.017. Epub 2006 Apr 11. Biol Psychiatry. 2006. PMID: 16581032 Review.
Cited by
-
Sitagliptin elevates plasma and CSF incretin levels following oral administration to nonhuman primates: relevance for neurodegenerative disorders.Geroscience. 2024 Oct;46(5):4397-4414. doi: 10.1007/s11357-024-01120-4. Epub 2024 Mar 27. Geroscience. 2024. PMID: 38532069 Free PMC article.
-
The Role of Microglia in the Development of Neurodegenerative Diseases.Biomedicines. 2021 Oct 12;9(10):1449. doi: 10.3390/biomedicines9101449. Biomedicines. 2021. PMID: 34680566 Free PMC article. Review.
-
PT320, a Sustained-Release GLP-1 Receptor Agonist, Ameliorates L-DOPA-Induced Dyskinesia in a Mouse Model of Parkinson's Disease.Int J Mol Sci. 2023 Feb 28;24(5):4687. doi: 10.3390/ijms24054687. Int J Mol Sci. 2023. PMID: 36902115 Free PMC article.
-
Attenuated Postprandial GLP-1 Response in Parkinson's Disease.Front Neurosci. 2021 Jul 2;15:660942. doi: 10.3389/fnins.2021.660942. eCollection 2021. Front Neurosci. 2021. PMID: 34276285 Free PMC article.
-
Circulating Dopamine Is Regulated by Dietary Glucose and Controls Glucagon-like 1 Peptide Action in White Adipose Tissue.Int J Mol Sci. 2023 Jan 27;24(3):2464. doi: 10.3390/ijms24032464. Int J Mol Sci. 2023. PMID: 36768789 Free PMC article.
References
-
- Ekstrand M. I.; Terzioglu M.; Galter D.; Zhu S.; Hofstetter C.; Lindqvist E.; Thams S.; Bergstrand A.; Hansson F. S.; Trifunovic A.; et al. (2007) Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc. Natl. Acad. Sci. U. S. A. 104, 1325–1330. 10.1073/pnas.0605208103. - DOI - PMC - PubMed
-
- Good C. H.; Hoffman A. F.; Hoffer B. J.; Chefer V. I.; Shippenberg T. S.; Bäckman C. M.; Larsson N. G.; Olson L.; Gellhaar S.; Galter D.; et al. (2011) Impaired nigrostriatal function precedes behavioral deficits in a genetic mitochondrial model of Parkinson’s disease. FASEB J. 25, 1333–1344. 10.1096/fj.10-173625. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
