Early detection of osteoarthritis in the rat with an antibody specific to type II collagen modified by reactive oxygen species
- PMID: 33853645
- PMCID: PMC8045329
- DOI: 10.1186/s13075-021-02502-1
Early detection of osteoarthritis in the rat with an antibody specific to type II collagen modified by reactive oxygen species
Abstract
Background: Osteoarthritis (OA) is a disease of the whole joint, with articular cartilage breakdown as a major characteristic. Inflammatory mediators, proteases, and oxidants produced by chondrocytes are known to be responsible for driving cartilage degradation. Nevertheless, the early pathogenic events are still unclear. To investigate this, we employed an antibody that is specific to oxidative post-translationally modified collagen type II (anti-oxPTM-CII) to detect early cartilage pathogenic changes in two rat models of OA.
Methods: The animals underwent surgery for destabilization of the medial meniscus (DMM) and were sacrificed after 3, 5, 7, 14, and 28 days. Alternatively, anterior cruciate ligament transection with partial meniscectomy (ACLT+pMx) was performed and animals were sacrificed after 1, 3, 5, 7, and 14 days. Joints were stained with toluidine blue and saffron du Gatinais for histological scoring, anti-oxPTM-CII, and anti-collagen type X antibodies (anti-CX).
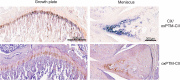
Results: We observed positive oxPTM-CII staining as early as 1 or 3 days after ACLT+pMx or DMM surgeries, respectively, before overt cartilage lesions were visible. oxPTM-CII was located mostly in the deep zone of the medial tibial cartilage, in the pericellular and territorial matrix of hypertrophic chondrocytes, and co-localized with CX staining. Staining was weak or absent for the lateral compartment or the contralateral knees except at later time points.
Conclusion: The results demonstrate that oxidant production and chondrocyte hypertrophy occur very early in the onset of OA, possibly initiating the pathogenic events of OA. We propose to use anti-oxPTM-CII as an early biomarker for OA ahead of radiographic changes.
Keywords: Collagen type II; Collagen type X; Hypertrophy; Osteoarthritis; Reactive oxygen species.
Conflict of interest statement
Anne Gigout, Sven Lindemann, Christian Brenneis, and Donata Harazin were employees of Merck KGaA at the time of the study. Anne Gigout and Didier Merciris are currently employees of Galapagos SASU. The other authors declare that they have no competing interests.
Figures



Similar articles
-
Detection and Evaluation of Serological Biomarkers to Predict Osteoarthritis in Anterior Cruciate Ligament Transection Combined Medial Meniscectomy Rat Model.Int J Mol Sci. 2021 Sep 22;22(19):10179. doi: 10.3390/ijms221910179. Int J Mol Sci. 2021. PMID: 34638520 Free PMC article.
-
Articular collagen degradation in the Hulth-Telhag model of osteoarthritis.Osteoarthritis Cartilage. 1999 Nov;7(6):539-47. doi: 10.1053/joca.1999.0258. Osteoarthritis Cartilage. 1999. PMID: 10558852
-
Anterior Cruciate Ligament Transection-Induced Cellular and Extracellular Events in Menisci: Implications for Osteoarthritis.Am J Sports Med. 2018 Apr;46(5):1185-1198. doi: 10.1177/0363546518756087. Epub 2018 Mar 7. Am J Sports Med. 2018. PMID: 29513553
-
Early and stable upregulation of collagen type II, collagen type I and YKL40 expression levels in cartilage during early experimental osteoarthritis occurs independent of joint location and histological grading.Arthritis Res Ther. 2005;7(1):R156-65. doi: 10.1186/ar1471. Epub 2004 Dec 7. Arthritis Res Ther. 2005. PMID: 15642136 Free PMC article.
-
[In vitro effect of alendronate on chondrocytes and articular cartilage and subchondral bone in rabbit anterior cruciate ligament transection model].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009 Dec;23(12):1474-81. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009. PMID: 20073314 Chinese.
Cited by
-
Dynamic weight-bearing test during jumping: A sensitive outcome measure of chronic osteoarthritis pain in rats.Heliyon. 2021 Sep 2;7(9):e07906. doi: 10.1016/j.heliyon.2021.e07906. eCollection 2021 Sep. Heliyon. 2021. PMID: 34522804 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

