Recombinant pseudorabies virus with gI/gE deletion generated by overlapping polymerase chain reaction and homologous recombination technology induces protection against the PRV variant PRV-GD2013
- PMID: 33853597
- PMCID: PMC8048318
- DOI: 10.1186/s12917-021-02861-6
Recombinant pseudorabies virus with gI/gE deletion generated by overlapping polymerase chain reaction and homologous recombination technology induces protection against the PRV variant PRV-GD2013
Abstract
Background: Since 2011, numerous highly virulent and antigenic variant viral strains have been reported in pigs that were vaccinated against the swine pseudorabies virus. These infections have led to substantial economic losses in the Chinese swine industry.
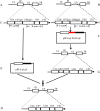
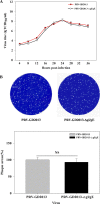
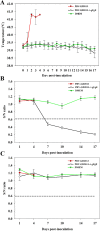
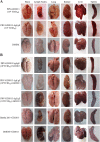
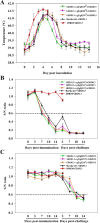
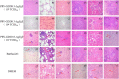
Results: This study, constructed a novel recombinant vaccine strain with gI/gE deletion (PRV-GD2013-ΔgI/gE) by overlapping PCR and homologous recombination technology. The growth curves and plaque morphology of the recombinant virus were similar to those of the parental strain. However, PRV-GD2013-ΔgI/gE infection was significantly attenuated in mice compared with that of PRV-GD2013. Two-week-old piglets had normal rectal temperatures and displayed no clinical symptoms after being inoculated with 105 TCID50 PRV-GD2013-ΔgI/gE, indicating that the recombinant virus was avirulent in piglets. Piglets were immunized with different doses of PRV-GD2013-ΔgI/gE, or a single dose of Bartha-K61 or DMEM, and infected with PRV-GD2013 at 14 days post-vaccination. Piglets given high doses of PRV-GD2013-ΔgI/gE showed no obvious clinical symptoms, and their antibody levels were higher than those of other groups, indicating that the piglets were completely protected from PRV-GD2013.
Conclusions: The PRV-GD2013-ΔgI/gE vaccine strain could be effective for immunizing Chinese swine herds against the pseudorabies virus (PRV) strain.
Keywords: PRV-GD2013-ΔgI/gE; Pseudorabies virus; Recombinant virus; Vaccine.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Safety and immunogenicity of an attenuated Chinese pseudorabies variant by dual deletion of TK&gE genes.BMC Vet Res. 2018 Sep 21;14(1):287. doi: 10.1186/s12917-018-1536-7. BMC Vet Res. 2018. PMID: 30241529 Free PMC article.
-
Pathogenicity and immunogenicity of a gI/gE/TK/UL13-gene-deleted variant pseudorabies virus strain in swine.Vet Microbiol. 2021 Jul;258:109104. doi: 10.1016/j.vetmic.2021.109104. Epub 2021 May 8. Vet Microbiol. 2021. PMID: 34004569
-
A live gI/gE-deleted pseudorabies virus (PRV) protects weaned piglets against lethal variant PRV challenge.Virus Genes. 2017 Aug;53(4):565-572. doi: 10.1007/s11262-017-1454-y. Epub 2017 Apr 17. Virus Genes. 2017. PMID: 28417300
-
Vaccines against pseudorabies virus (PrV).Vet Microbiol. 2017 Jul;206:3-9. doi: 10.1016/j.vetmic.2016.11.019. Epub 2016 Nov 18. Vet Microbiol. 2017. PMID: 27890448 Review.
-
An overview of live attenuated recombinant pseudorabies viruses for use as novel vaccines.J Immunol Res. 2014;2014:824630. doi: 10.1155/2014/824630. Epub 2014 Jun 5. J Immunol Res. 2014. PMID: 24995348 Free PMC article. Review.
Cited by
-
Pseudorabies Virus Glycoproteins E and B Application in Vaccine and Diagnosis Kit Development.Vaccines (Basel). 2024 Sep 20;12(9):1078. doi: 10.3390/vaccines12091078. Vaccines (Basel). 2024. PMID: 39340108 Free PMC article. Review.
-
Efficacy of a gB + gD-based subunit vaccine and the adjuvant granulocyte-macrophage colony stimulating factor for pseudorabies virus in rabbits.Front Microbiol. 2022 Aug 3;13:965997. doi: 10.3389/fmicb.2022.965997. eCollection 2022. Front Microbiol. 2022. PMID: 35992660 Free PMC article.
-
The Deletion of US3 Gene of Pseudorabies Virus (PRV) ΔgE/TK Strain Induces Increased Immunogenicity in Mice.Vaccines (Basel). 2022 Sep 23;10(10):1603. doi: 10.3390/vaccines10101603. Vaccines (Basel). 2022. PMID: 36298468 Free PMC article.
-
The Attenuated Pseudorabies Virus Vaccine Strain Bartha Hyperactivates Plasmacytoid Dendritic Cells by Generating Large Amounts of Cell-Free Virus in Infected Epithelial Cells.J Virol. 2022 Jun 22;96(12):e0219921. doi: 10.1128/jvi.02199-21. Epub 2022 May 23. J Virol. 2022. PMID: 35604216 Free PMC article.
-
Coinfection of Porcine Circovirus 2 and Pseudorabies Virus Enhances Immunosuppression and Inflammation through NF-κB, JAK/STAT, MAPK, and NLRP3 Pathways.Int J Mol Sci. 2022 Apr 18;23(8):4469. doi: 10.3390/ijms23084469. Int J Mol Sci. 2022. PMID: 35457287 Free PMC article.
References
-
- Wittmann G, Rziha HJ. Aujeszky’s disease (Pseudorabies) in pigs. Dev Vet Virol. 1989;9:230–325. doi: 10.1007/978-1-4613-1587-2_7. - DOI
MeSH terms
Substances
Grants and funding
- 2016YFD0500700,2017YFD0500600/National Key Research Development Program of China
- 201803020005/Science and Technology Program of Guangzhou, China
- 31672590, U1405216, 31472200/National Natural Science Foundation of China
- Nos. 2019B02021103/Science and Technology Planning Project of Guangdong Province of China
LinkOut - more resources
Full Text Sources
Other Literature Sources

