Therapeutic B-cell depletion reverses progression of Alzheimer's disease
- PMID: 33846335
- PMCID: PMC8042032
- DOI: 10.1038/s41467-021-22479-4
Therapeutic B-cell depletion reverses progression of Alzheimer's disease
Abstract
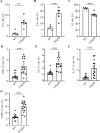
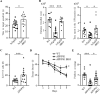
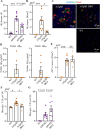
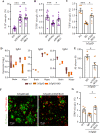
The function of B cells in Alzheimer's disease (AD) is not fully understood. While immunoglobulins that target amyloid beta (Aβ) may interfere with plaque formation and hence progression of the disease, B cells may contribute beyond merely producing immunoglobulins. Here we show that AD is associated with accumulation of activated B cells in circulation, and with infiltration of B cells into the brain parenchyma, resulting in immunoglobulin deposits around Aβ plaques. Using three different murine transgenic models, we provide counterintuitive evidence that the AD progression requires B cells. Despite expression of the AD-fostering transgenes, the loss of B cells alone is sufficient to reduce Aβ plaque burden and disease-associated microglia. It reverses behavioral and memory deficits and restores TGFβ+ microglia, respectively. Moreover, therapeutic depletion of B cells at the onset of the disease retards AD progression in mice, suggesting that targeting B cells may also benefit AD patients.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Human intravenous immunoglobulin provides protection against Aβ toxicity by multiple mechanisms in a mouse model of Alzheimer's disease.J Neuroinflammation. 2010 Dec 7;7:90. doi: 10.1186/1742-2094-7-90. J Neuroinflammation. 2010. PMID: 21138577 Free PMC article.
-
Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models.Elife. 2020 Jun 8;9:e54083. doi: 10.7554/eLife.54083. Elife. 2020. PMID: 32510331 Free PMC article.
-
Human APOE4 increases microglia reactivity at Aβ plaques in a mouse model of Aβ deposition.J Neuroinflammation. 2014 Jun 19;11:111. doi: 10.1186/1742-2094-11-111. J Neuroinflammation. 2014. PMID: 24948358 Free PMC article.
-
Multiple inflammatory profiles of microglia and altered neuroimages in APP/PS1 transgenic AD mice.Brain Res Bull. 2020 Mar;156:86-104. doi: 10.1016/j.brainresbull.2020.01.003. Epub 2020 Jan 10. Brain Res Bull. 2020. PMID: 31931120
-
Microglia contributes to plaque growth by cell death due to uptake of amyloid β in the brain of Alzheimer's disease mouse model.Glia. 2016 Dec;64(12):2274-2290. doi: 10.1002/glia.23074. Epub 2016 Sep 23. Glia. 2016. PMID: 27658617
Cited by
-
Roles in Innate Immunity.Adv Neurobiol. 2024;37:263-286. doi: 10.1007/978-3-031-55529-9_15. Adv Neurobiol. 2024. PMID: 39207697 Review.
-
Identification of diagnostic signatures associated with immune infiltration in Alzheimer's disease by integrating bioinformatic analysis and machine-learning strategies.Front Aging Neurosci. 2022 Jul 29;14:919614. doi: 10.3389/fnagi.2022.919614. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35966794 Free PMC article.
-
Current views on meningeal lymphatics and immunity in aging and Alzheimer's disease.Mol Neurodegener. 2023 Aug 14;18(1):55. doi: 10.1186/s13024-023-00645-0. Mol Neurodegener. 2023. PMID: 37580702 Free PMC article. Review.
-
CXCR6 orchestrates brain CD8+ T cell residency and limits mouse Alzheimer's disease pathology.Nat Immunol. 2023 Oct;24(10):1735-1747. doi: 10.1038/s41590-023-01604-z. Epub 2023 Sep 7. Nat Immunol. 2023. PMID: 37679549 Free PMC article.
-
Enrichment of liver MAIT cells in a mouse model of Alzheimer's disease.J Neuroimmunol. 2024 May 15;390:578332. doi: 10.1016/j.jneuroim.2024.578332. Epub 2024 Mar 21. J Neuroimmunol. 2024. PMID: 38537322
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

