Positive allosteric modulation of the mu-opioid receptor produces analgesia with reduced side effects
- PMID: 33846240
- PMCID: PMC8072371
- DOI: 10.1073/pnas.2000017118
Positive allosteric modulation of the mu-opioid receptor produces analgesia with reduced side effects
Abstract
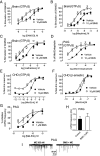
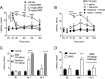
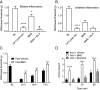
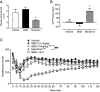
Positive allosteric modulators (PAMs) of the mu-opioid receptor (MOR) have been hypothesized as potentially safer analgesics than traditional opioid drugs. This is based on the idea that PAMs will promote the action of endogenous opioid peptides while preserving their temporal and spatial release patterns and so have an improved therapeutic index. However, this hypothesis has never been tested. Here, we show that a mu-PAM, BMS-986122, enhances the ability of the endogenous opioid Methionine-enkephalin (Met-Enk) to stimulate G protein activity in mouse brain homogenates without activity on its own and to enhance G protein activation to a greater extent than β-arrestin recruitment in Chinese hamster ovary (CHO) cells expressing human mu-opioid receptors. Moreover, BMS-986122 increases the potency of Met-Enk to inhibit GABA release in the periaqueductal gray, an important site for antinociception. We describe in vivo experiments demonstrating that the mu-PAM produces antinociception in mouse models of acute noxious heat pain as well as inflammatory pain. These effects are blocked by MOR antagonists and are consistent with the hypothesis that in vivo mu-PAMs enhance the activity of endogenous opioid peptides. Because BMS-986122 does not bind to the orthosteric site and has no inherent agonist action at endogenously expressed levels of MOR, it produces a reduced level of morphine-like side effects of constipation, reward as measured by conditioned place preference, and respiratory depression. These data provide a rationale for the further exploration of the action and safety of mu-PAMs as an innovative approach to pain management.
Keywords: allostery; analgesia; endogenous opioid peptides; mu-opioid receptor; signaling bias.
Conflict of interest statement
Competing interest statement: N.T.B. and A.A. have a patent related to this work: “Positive allosteric modulators and silent allosteric modulators of the μ-opioid receptor.” Publication number: WO2014/107344 A1. Publication date: July 10, 2014.
Figures

Comment in
-
Do positive allosteric modulators (PAMs) of the MOR exert antinociception with reduced side effects under pathological conditions?Proc Natl Acad Sci U S A. 2021 Jul 13;118(28):e2107784118. doi: 10.1073/pnas.2107784118. Proc Natl Acad Sci U S A. 2021. PMID: 34260379 Free PMC article. No abstract available.
-
Reply to Zhuang et al.: Potential side effects of positive allosteric modulators of the mu-opioid receptor.Proc Natl Acad Sci U S A. 2021 Jul 13;118(28):e2108493118. doi: 10.1073/pnas.2108493118. Proc Natl Acad Sci U S A. 2021. PMID: 34260391 Free PMC article. No abstract available.
Similar articles
-
Disruption of the Na+ ion binding site as a mechanism for positive allosteric modulation of the mu-opioid receptor.Proc Natl Acad Sci U S A. 2014 Dec 23;111(51):18369-74. doi: 10.1073/pnas.1415013111. Epub 2014 Dec 8. Proc Natl Acad Sci U S A. 2014. PMID: 25489080 Free PMC article.
-
Positive allosteric modulation of the cannabinoid type-1 receptor (CB1R) in periaqueductal gray (PAG) antagonizes anti-nociceptive and cellular effects of a mu-opioid receptor agonist in morphine-withdrawn rats.Psychopharmacology (Berl). 2020 Dec;237(12):3729-3739. doi: 10.1007/s00213-020-05650-5. Epub 2020 Aug 28. Psychopharmacology (Berl). 2020. PMID: 32857187 Free PMC article.
-
Positive allosteric modulators of the μ-opioid receptor: a novel approach for future pain medications.Br J Pharmacol. 2015 Jan;172(2):277-86. doi: 10.1111/bph.12599. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24460691 Free PMC article. Review.
-
Discovery of positive allosteric modulators and silent allosteric modulators of the μ-opioid receptor.Proc Natl Acad Sci U S A. 2013 Jun 25;110(26):10830-5. doi: 10.1073/pnas.1300393110. Epub 2013 Jun 10. Proc Natl Acad Sci U S A. 2013. PMID: 23754417 Free PMC article.
-
Ligand-Free Signaling of G-Protein-Coupled Receptors: Relevance to μ Opioid Receptors in Analgesia and Addiction.Molecules. 2022 Sep 8;27(18):5826. doi: 10.3390/molecules27185826. Molecules. 2022. PMID: 36144565 Free PMC article. Review.
Cited by
-
Signaling Modulation Mediated by Ligand Water Interactions with the Sodium Site at μOR.ACS Cent Sci. 2024 Jul 17;10(8):1490-1503. doi: 10.1021/acscentsci.4c00525. eCollection 2024 Aug 28. ACS Cent Sci. 2024. PMID: 39220695 Free PMC article.
-
Coincident Regulation of PLCβ Signaling by Gq-Coupled and μ-Opioid Receptors Opposes Opioid-Mediated Antinociception.Mol Pharmacol. 2022 Dec;102(6):269-279. doi: 10.1124/molpharm.122.000541. Epub 2022 Sep 18. Mol Pharmacol. 2022. PMID: 36116788 Free PMC article.
-
Advances in attenuating opioid-induced respiratory depression: A narrative review.Medicine (Baltimore). 2024 Jul 19;103(29):e38837. doi: 10.1097/MD.0000000000038837. Medicine (Baltimore). 2024. PMID: 39029082 Free PMC article. Review.
-
Application of Mixed-Solvent Molecular Dynamics Simulations for Prediction of Allosteric Sites on G Protein-Coupled Receptors.Mol Pharmacol. 2023 May;103(5):274-285. doi: 10.1124/molpharm.122.000612. Epub 2023 Mar 3. Mol Pharmacol. 2023. PMID: 36868791 Free PMC article.
-
Biased, Bitopic, Opioid-Adrenergic Tethered Compounds May Improve Specificity, Lower Dosage and Enhance Agonist or Antagonist Function with Reduced Risk of Tolerance and Addiction.Pharmaceuticals (Basel). 2022 Feb 10;15(2):214. doi: 10.3390/ph15020214. Pharmaceuticals (Basel). 2022. PMID: 35215326 Free PMC article. Review.
References
-
- Mansour A., Fox C. A., Thompson R. C., Akil H., Watson S. J., mu-Opioid receptor mRNA expression in the rat CNS: Comparison to mu-receptor binding. Brain Res. 643, 245–265 (1994). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

