Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition
- PMID: 33845942
- PMCID: PMC8043748
- DOI: 10.7554/eLife.59826
Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition
Abstract
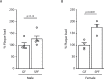
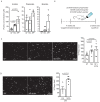
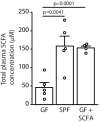
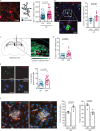
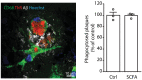
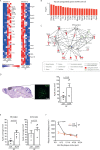
Previous studies have identified a crucial role of the gut microbiome in modifying Alzheimer's disease (AD) progression. However, the mechanisms of microbiome-brain interaction in AD were so far unknown. Here, we identify microbiota-derived short chain fatty acids (SCFA) as microbial metabolites which promote Aβ deposition. Germ-free (GF) AD mice exhibit a substantially reduced Aβ plaque load and markedly reduced SCFA plasma concentrations; conversely, SCFA supplementation to GF AD mice increased the Aβ plaque load to levels of conventionally colonized (specific pathogen-free [SPF]) animals and SCFA supplementation to SPF mice even further exacerbated plaque load. This was accompanied by the pronounced alterations in microglial transcriptomic profile, including upregulation of ApoE. Despite increased microglial recruitment to Aβ plaques upon SCFA supplementation, microglia contained less intracellular Aβ. Taken together, our results demonstrate that microbiota-derived SCFA are critical mediators along the gut-brain axis which promote Aβ deposition likely via modulation of the microglial phenotype.
Keywords: alzheimer's disease; amyloid; immunology; inflammation; metabolites; microbiome; microglia; mouse; neuroinflammation; neuroscience.
© 2021, Colombo et al.
Conflict of interest statement
AC, RS, GL, VS, SR, SH, LS, AV, FP, SP, FK, MG, EW, JH, CB, MD, HS, MG, CH, ST, AL No competing interests declared, AM Reviewing editor, eLife
Figures



Similar articles
-
Sodium oligomannate alters gut microbiota, reduces cerebral amyloidosis and reactive microglia in a sex-specific manner.Mol Neurodegener. 2024 Feb 17;19(1):18. doi: 10.1186/s13024-023-00700-w. Mol Neurodegener. 2024. PMID: 38365827 Free PMC article.
-
The gut microbiome regulates astrocyte reaction to Aβ amyloidosis through microglial dependent and independent mechanisms.Mol Neurodegener. 2023 Jul 6;18(1):45. doi: 10.1186/s13024-023-00635-2. Mol Neurodegener. 2023. PMID: 37415149 Free PMC article.
-
Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models.Elife. 2020 Jun 8;9:e54083. doi: 10.7554/eLife.54083. Elife. 2020. PMID: 32510331 Free PMC article.
-
Protection against Alzheimer's disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis.Biofactors. 2021 Mar;47(2):218-231. doi: 10.1002/biof.1703. Epub 2020 Dec 21. Biofactors. 2021. PMID: 33347668 Review.
-
Tryptophan Metabolism in Alzheimer's Disease with the Involvement of Microglia and Astrocyte Crosstalk and Gut-Brain Axis.Aging Dis. 2024 Oct 1;15(5):2168-2190. doi: 10.14336/AD.2024.0134. Aging Dis. 2024. PMID: 38916729 Free PMC article. Review.
Cited by
-
Deciphering sources of PET signals in the tumor microenvironment of glioblastoma at cellular resolution.Sci Adv. 2023 Oct 27;9(43):eadi8986. doi: 10.1126/sciadv.adi8986. Epub 2023 Oct 27. Sci Adv. 2023. PMID: 37889970 Free PMC article.
-
The Gut Microbiota and Short-Chain Fatty Acids Profile in Postural Orthostatic Tachycardia Syndrome.Front Physiol. 2022 Jun 2;13:879012. doi: 10.3389/fphys.2022.879012. eCollection 2022. Front Physiol. 2022. PMID: 35733987 Free PMC article.
-
SCFAs promote intestinal double-negative T cells to regulate the inflammatory response mediated by NLRP3 inflammasome.Aging (Albany NY). 2021 Sep 7;13(17):21470-21482. doi: 10.18632/aging.203487. Epub 2021 Sep 7. Aging (Albany NY). 2021. PMID: 34491906 Free PMC article.
-
Reverse engineering the Gut-Brain Axis and microbiome-metabolomics for symbiotic/pathogenic balance in neurodegenerative diseases.Gut Microbes. 2024 Jan-Dec;16(1):2422468. doi: 10.1080/19490976.2024.2422468. Epub 2024 Nov 10. Gut Microbes. 2024. PMID: 39523450 Free PMC article. Review.
-
Regulation of microglial physiology by the microbiota.Gut Microbes. 2022 Jan-Dec;14(1):2125739. doi: 10.1080/19490976.2022.2125739. Gut Microbes. 2022. PMID: 36151874 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

