A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: A broad spectrum antiviral candidate for treatment of COVID-19
- PMID: 33842908
- PMCID: PMC8021896
- DOI: 10.1016/j.lanepe.2021.100084
A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: A broad spectrum antiviral candidate for treatment of COVID-19
Abstract
Background: Coronavirus disease 19 (COVID-19) is spreading globally and treatment options remain limited. A formulation of niclosamide, a potent anti-SARS-CoV-2 agent and a broad-spectrum antiviral treatment candidate, optimized for inhalation and intranasal administration (UNI91104) was developed.
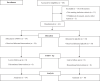
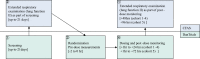
Methods: We conducted a randomized, placebo-controlled, double-blind, single-centre, dose-ascending Phase 1 trial to assess the safety of UNI91104 in Denmark (NCT04576312). Healthy volunteers were randomly assigned to a ascending single dose in cohort 1-4 and five doses over 2.5 days in cohort 5. Inclusion criteria included a minimum 80% of predicted lung function. Exclusion criteria included severe, clinically significant allergies and current acute or chronic condition especially airway diseases. Safety was evaluated through adverse events (AEs) and pulmonary function tests including forced expiratory volume in one second (FEV1) and fractional exhaled nitric oxide (FeNO) tests. The primary endpoints were defined as the frequency of reported AEs and the change of safety variables relative to pre-dose. Data from all enroled healthy volunteers receiving any amount of IMP was included in the primary analyses. The pharmacokinetics of UNI91104 was determined.
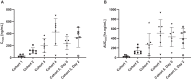
Findings: The trial was conducted between 29 June 2020 and 08 August 2020. Thirty-four healthy volunteers received UNI91104 and ten placebo. No serious AEs or discontinuation were reported. Mild irritation in the upper respiratory tract following inhalation of UNI91104 was reported as most frequent AE (45 events in 26 healthy volunteers, 59% of all healthy volunteers). Nasal application was well-tolerated. There was no evidence of difference in the change of mean levels of pulmonary function tests between active and placebo group across all cohorts. Five healthy volunteers (11.4%) (1 on placebo) had signs of increased transient FeNO and 4 on active (9.1%) experienced asymptomatic drops in FEV1, which resolved spontaneously or were reversible with a β2-agonist. Niclosamide exhibited dose-proportional pharmacokinetics following inhalation and intranasal administration.
Interpretation: UNI91104, a promising candidate for inhalation and intranasal therapy against COVID-19 and other viral respiratory tract infections is well-tolerated in healthy volunteers and warrants further testing in patient trials.
Funding: The study was funded by Innovationsfonden Denmark and UNION therapeutics.
© 2021 The Authors.
Conflict of interest statement
Dr. Sommer, Ms. Weiss and Mr Jellingsø are shareholders or benefit from an employee incentive scheme in UNION therapeutics. All other authors have nothing to disclose.
Figures
Similar articles
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Tiotropium bromide. A review of its use as maintenance therapy in patients with COPD.Treat Respir Med. 2004;3(4):247-68. doi: 10.2165/00151829-200403040-00005. Treat Respir Med. 2004. PMID: 15350163 Review.
-
Leukotriene receptor antagonists in children with cystic fibrosis lung disease : anti-inflammatory and clinical effects.Paediatr Drugs. 2005;7(6):353-63. doi: 10.2165/00148581-200507060-00004. Paediatr Drugs. 2005. PMID: 16356023 Review.
Cited by
-
In vitro synergistic antiviral activity of repurposed drugs against enterovirus 71.Arch Virol. 2024 Jul 30;169(8):169. doi: 10.1007/s00705-024-06097-1. Arch Virol. 2024. PMID: 39078431
-
In Vitro Evaluation and Mitigation of Niclosamide's Liabilities as a COVID-19 Treatment.bioRxiv [Preprint]. 2022 Jul 13:2022.06.24.497526. doi: 10.1101/2022.06.24.497526. bioRxiv. 2022. Update in: Vaccines (Basel). 2022 Aug 09;10(8):1284. doi: 10.3390/vaccines10081284. PMID: 35860224 Free PMC article. Updated. Preprint.
-
Host directed therapies: COVID-19 and beyond.Curr Res Pharmacol Drug Discov. 2021;2:100058. doi: 10.1016/j.crphar.2021.100058. Epub 2021 Sep 25. Curr Res Pharmacol Drug Discov. 2021. PMID: 34870156 Free PMC article. Review.
-
In Vitro Evaluation and Mitigation of Niclosamide's Liabilities as a COVID-19 Treatment.Vaccines (Basel). 2022 Aug 9;10(8):1284. doi: 10.3390/vaccines10081284. Vaccines (Basel). 2022. PMID: 36016172 Free PMC article.
-
Getting hands on a drug for Covid-19: Inhaled and Intranasal Niclosamide.Lancet Reg Health Eur. 2021 May;4:100094. doi: 10.1016/j.lanepe.2021.100094. Epub 2021 Apr 13. Lancet Reg Health Eur. 2021. PMID: 33870260 Free PMC article. No abstract available.
References
-
- World Health Organization . WHO; 2020. Novel coronavirus (COVID-19) situation.
-
- NIH. COVID-19 Treatment Guidelines Panel . National Institutes of Health (NIH); 2020. Coronavirus disease 2019 (COVID-19) treatment guidelines. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

