SLI1 confers broad-spectrum resistance to phloem-feeding insects
- PMID: 33837973
- PMCID: PMC8360143
- DOI: 10.1111/pce.14064
SLI1 confers broad-spectrum resistance to phloem-feeding insects
Abstract
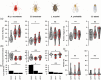
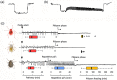
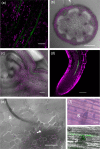
Resistance (R) genes usually compete in a coevolutionary arms race with reciprocal effectors to confer strain-specific resistance to pathogens or herbivorous insects. Here, we investigate the specificity of SLI1, a recently identified R gene in Arabidopsis that encodes a small heat shock-like protein involved in resistance to Myzus persicae aphids. In a panel with several aphid and whitefly species, SLI1 compromised reproductive rates of three species: the tobacco aphid M. persicae nicotianae, the cabbage aphid Brevicoryne brassicae and the cabbage whitefly Aleyrodes proletella. Electrical penetration graph recording of aphid behaviour, revealed shorter salivations and a 3-to-5-fold increase in phloem feeding on sli1 loss-of-function plants. The mustard aphid Lipaphis erysimi and Bemisia tabaci whitefly were not affected by SLI1. Unlike the other two aphid species, L. erysimi exhibited repetitive salivations preceding successful phloem feeding, indicating a role of salivary effectors in overcoming SLI1-mediated resistance. Microscopic characterization showed that SLI1 proteins localize in the sieve tubes of virtually all above- and below-ground tissues and co-localize with the aphid stylet tip after penetration of the sieve element plasma membrane. These observations reveal an unconventional R gene that escapes the paradigm of strain specificity and confers broad-spectrum quantitative resistance to phloem-feeding insects.
Keywords: R genes; aphids; phloem; plant resistance; whiteflies.
© 2021 The Authors. Plant, Cell & Environment published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Harpin-induced expression and transgenic overexpression of the phloem protein gene AtPP2-A1 in Arabidopsis repress phloem feeding of the green peach aphid Myzus persicae.BMC Plant Biol. 2011 Jan 13;11:11. doi: 10.1186/1471-2229-11-11. BMC Plant Biol. 2011. PMID: 21226963 Free PMC article.
-
SIEVE ELEMENT-LINING CHAPERONE1 Restricts Aphid Feeding on Arabidopsis during Heat Stress.Plant Cell. 2017 Oct;29(10):2450-2464. doi: 10.1105/tpc.16.00424. Epub 2017 Sep 28. Plant Cell. 2017. PMID: 28970334 Free PMC article.
-
HrpN Ea-induced deterrent effect on phloem feeding of the green peach aphid Myzus persicae requires AtGSL5 and AtMYB44 genes in Arabidopsis thaliana.J Biosci. 2011 Mar;36(1):123-37. doi: 10.1007/s12038-011-9016-2. J Biosci. 2011. PMID: 21451254
-
Biochemistry and molecular biology of Arabidopsis-aphid interactions.Bioessays. 2007 Sep;29(9):871-83. doi: 10.1002/bies.20624. Bioessays. 2007. PMID: 17691101 Review.
-
Defenses against Virus and Vector: A Phloem-Biological Perspective on RTM- and SLI1-Mediated Resistance to Potyviruses and Aphids.Viruses. 2020 Jan 22;12(2):129. doi: 10.3390/v12020129. Viruses. 2020. PMID: 31979012 Free PMC article. Review.
Cited by
-
Effects of condensed tannins on behavior and performance of a specialist aphid on aspen.Ecol Evol. 2022 Aug 23;12(8):e9229. doi: 10.1002/ece3.9229. eCollection 2022 Aug. Ecol Evol. 2022. PMID: 36016819 Free PMC article.
-
Common resistance mechanisms are deployed by plants against sap-feeding herbivorous insects: insights from a meta-analysis and systematic review.Sci Rep. 2022 Oct 25;12(1):17836. doi: 10.1038/s41598-022-20741-3. Sci Rep. 2022. PMID: 36284143 Free PMC article.
-
Comparative Plant Transcriptome Profiling of Arabidopsis thaliana Col-0 and Camelina sativa var. Celine Infested with Myzus persicae Aphids Acquiring Circulative and Noncirculative Viruses Reveals Virus- and Plant-Specific Alterations Relevant to Aphid Feeding Behavior and Transmission.Microbiol Spectr. 2022 Aug 31;10(4):e0013622. doi: 10.1128/spectrum.00136-22. Epub 2022 Jul 20. Microbiol Spectr. 2022. PMID: 35856906 Free PMC article.
-
The sieve-element endoplasmic reticulum: A focal point of phytoplasma-host plant interaction?Front Microbiol. 2023 Feb 2;14:1030414. doi: 10.3389/fmicb.2023.1030414. eCollection 2023. Front Microbiol. 2023. PMID: 36819061 Free PMC article.
-
Current status of molecular rice breeding for durable and broad-spectrum resistance to major diseases and insect pests.Theor Appl Genet. 2024 Sep 10;137(10):219. doi: 10.1007/s00122-024-04729-3. Theor Appl Genet. 2024. PMID: 39254868 Free PMC article. Review.
References
-
- Adler D. & Kelly S.T. (2020) Vioplot: Violin plot.
-
- Bakthisaran, R., Tangirala, R., & Rao, C. M. (2015). Small heat shock proteins: Role in cellular functions and pathology. Biochimica et Biophysica Acta (BBA)—Proteins and Proteomics, 1854, 291–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

