Zinc transporter mutations linked to acrodermatitis enteropathica disrupt function and cause mistrafficking
- PMID: 33837739
- PMCID: PMC7949036
- DOI: 10.1016/j.jbc.2021.100269
Zinc transporter mutations linked to acrodermatitis enteropathica disrupt function and cause mistrafficking
Abstract
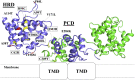
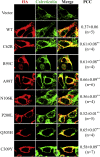
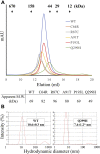
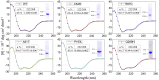
ZIP4 is a representative member of the Zrt-/Irt-like protein (ZIP) transporter family and responsible for zinc uptake from diet. Loss-of-function mutations of human ZIP4 (hZIP4) drastically reduce zinc absorption, causing a life-threatening autosomal recessive disorder, acrodermatitis enteropathica (AE). These mutations occur not only in the conserved transmembrane zinc transport machinery, but also in the extracellular domain (ECD) of hZIP4, which is only present in a fraction of mammalian ZIPs. How these AE-causing ECD mutations lead to ZIP4 malfunction has not be fully clarified. In this work, we characterized all seven confirmed AE-causing missense mutations in hZIP4-ECD and found that the variants exhibited completely abolished zinc transport activity in a cell-based transport assay. Although the variants were able to be expressed in HEK293T cells, they failed to traffic to the cell surface and were largely retained in the ER with immature glycosylation. When the corresponding mutations were introduced in the ECD of ZIP4 from Pteropus Alecto, a close homolog of hZIP4, the variants exhibited structural defects or reduced thermal stability, which likely accounts for intracellular mistrafficking of the AE-associated variants and as such a total loss of zinc uptake activity. This work provides a molecular pathogenic mechanism for AE.
Keywords: ZIP4; acrodermatitis enteropathica; disease-causing mutation; extracellular domain; misfolding; mistrafficking; zinc transporter.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflicts of interest with the contents of this article.
Figures



Similar articles
-
The histidine-rich loop in the extracellular domain of ZIP4 binds zinc and plays a role in zinc transport.Biochem J. 2019 Jun 28;476(12):1791-1803. doi: 10.1042/BCJ20190108. Biochem J. 2019. PMID: 31164399 Free PMC article.
-
Acrodermatitis enteropathica mutations affect transport activity, localization and zinc-responsive trafficking of the mouse ZIP4 zinc transporter.Hum Mol Genet. 2004 Mar 1;13(5):563-71. doi: 10.1093/hmg/ddh049. Epub 2004 Jan 6. Hum Mol Genet. 2004. PMID: 14709598
-
Regulation and function of Zip4, the acrodermatitis enteropathica gene.Biochem Soc Trans. 2008 Dec;36(Pt 6):1242-6. doi: 10.1042/BST0361242. Biochem Soc Trans. 2008. PMID: 19021533 Free PMC article. Review.
-
Elucidating the H+ Coupled Zn2+ Transport Mechanism of ZIP4; Implications in Acrodermatitis Enteropathica.Int J Mol Sci. 2020 Jan 22;21(3):734. doi: 10.3390/ijms21030734. Int J Mol Sci. 2020. PMID: 31979155 Free PMC article.
-
Genetic causes and gene–nutrient interactions in mammalian zinc deficiencies: acrodermatitis enteropathica and transient neonatal zinc deficiency as examples.J Trace Elem Med Biol. 2015 Jan;29:47-62. doi: 10.1016/j.jtemb.2014.10.003. J Trace Elem Med Biol. 2015. PMID: 25468189 Review.
Cited by
-
Sophisticated expression responses of ZNT1 and MT in response to changes in the expression of ZIPs.Sci Rep. 2022 May 5;12(1):7334. doi: 10.1038/s41598-022-10925-2. Sci Rep. 2022. PMID: 35513474 Free PMC article.
-
Nutritional Supplements for the Treatment of Neuropathic Pain.Biomedicines. 2021 Jun 13;9(6):674. doi: 10.3390/biomedicines9060674. Biomedicines. 2021. PMID: 34199290 Free PMC article. Review.
-
The Molecular Basis for Zinc Bioavailability.Int J Mol Sci. 2023 Mar 31;24(7):6561. doi: 10.3390/ijms24076561. Int J Mol Sci. 2023. PMID: 37047530 Free PMC article. Review.
-
The Influence of Dietary Supplementations on Neuropathic Pain.Life (Basel). 2022 Jul 27;12(8):1125. doi: 10.3390/life12081125. Life (Basel). 2022. PMID: 36013304 Free PMC article. Review.
-
Rational engineering of an elevator-type metal transporter ZIP8 reveals a conditional selectivity filter critically involved in determining substrate specificity.Commun Biol. 2023 Jul 26;6(1):778. doi: 10.1038/s42003-023-05146-w. Commun Biol. 2023. PMID: 37495662 Free PMC article.
References
-
- McCall K.A., Huang C., Fierke C.A. Function and mechanism of zinc metalloenzymes. J. Nutr. 2000;130:1437S–1446S. - PubMed
-
- Andreini C., Banci L., Bertini I., Rosato A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006;5:196–201. - PubMed
-
- Maret W., Sandstead H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006;20:3–18. - PubMed
-
- Kury S., Dreno B., Bezieau S., Giraudet S., Kharfi M., Kamoun R., Moisan J.P. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat. Genet. 2002;31:239–240. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

