SLPI is a critical mediator that controls PTH-induced bone formation
- PMID: 33837198
- PMCID: PMC8035405
- DOI: 10.1038/s41467-021-22402-x
SLPI is a critical mediator that controls PTH-induced bone formation
Abstract
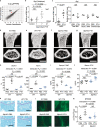
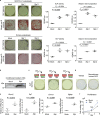
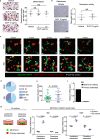
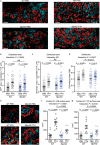
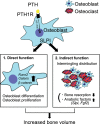
Osteoclastic bone resorption and osteoblastic bone formation/replenishment are closely coupled in bone metabolism. Anabolic parathyroid hormone (PTH), which is commonly used for treating osteoporosis, shifts the balance from osteoclastic to osteoblastic, although it is unclear how these cells are coordinately regulated by PTH. Here, we identify a serine protease inhibitor, secretory leukocyte protease inhibitor (SLPI), as a critical mediator that is involved in the PTH-mediated shift to the osteoblastic phase. Slpi is highly upregulated in osteoblasts by PTH, while genetic ablation of Slpi severely impairs PTH-induced bone formation. Slpi induction in osteoblasts enhances its differentiation, and increases osteoblast-osteoclast contact, thereby suppressing osteoclastic function. Intravital bone imaging reveals that the PTH-mediated association between osteoblasts and osteoclasts is disrupted in the absence of SLPI. Collectively, these results demonstrate that SLPI regulates the communication between osteoblasts and osteoclasts to promote PTH-induced bone anabolism.
Conflict of interest statement
T.Y. and R.T.-K. are full-time employees of Asahi Kasei Pharma Corporation. The other authors have no conflict of interest.
Figures
Similar articles
-
The Notch Ligand Jagged1 Regulates the Osteoblastic Lineage by Maintaining the Osteoprogenitor Pool.J Bone Miner Res. 2017 Jun;32(6):1320-1331. doi: 10.1002/jbmr.3106. Epub 2017 Mar 9. J Bone Miner Res. 2017. PMID: 28277610 Free PMC article.
-
Absence of bone sialoprotein (BSP) impairs primary bone formation and resorption: the marrow ablation model under PTH challenge.Bone. 2012 May;50(5):1064-73. doi: 10.1016/j.bone.2012.02.014. Bone. 2012. PMID: 22586700
-
Over-expression of TIMP-1 in osteoblasts increases the anabolic response to PTH.Bone. 2007 Jan;40(1):75-83. doi: 10.1016/j.bone.2006.07.013. Epub 2006 Sep 1. Bone. 2007. PMID: 16949899
-
[Biological function of bone cells on the PTH-driven anabolic effect].Clin Calcium. 2012 Mar;22(3):373-9. Clin Calcium. 2012. PMID: 22370304 Review. Japanese.
-
[Bone histology after intermittent PTH treatment - animal models -].Clin Calcium. 2013 Mar;23(3):347-53. Clin Calcium. 2013. PMID: 23445886 Review. Japanese.
Cited by
-
Osteoclasts adapt to physioxia perturbation through DNA demethylation.EMBO Rep. 2021 Dec 6;22(12):e53035. doi: 10.15252/embr.202153035. Epub 2021 Oct 18. EMBO Rep. 2021. PMID: 34661337 Free PMC article.
-
Breast Cancer with Bone Metastasis: Molecular Insights and Clinical Management.Cells. 2021 Jun 2;10(6):1377. doi: 10.3390/cells10061377. Cells. 2021. PMID: 34199522 Free PMC article. Review.
-
The secretory leukocyte protease inhibitor (SLPI) in pathophysiology of non-communicable diseases: Evidence from experimental studies to clinical applications.Heliyon. 2024 Jan 17;10(2):e24550. doi: 10.1016/j.heliyon.2024.e24550. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38312697 Free PMC article. Review.
-
Determination of the physiological range of oxygen tension in bone marrow monocytes using two-photon phosphorescence lifetime imaging microscopy.Sci Rep. 2022 Mar 10;12(1):3497. doi: 10.1038/s41598-022-07521-9. Sci Rep. 2022. PMID: 35273210 Free PMC article.
-
Connection between Osteoarthritis and Nitric Oxide: From Pathophysiology to Therapeutic Target.Molecules. 2023 Feb 9;28(4):1683. doi: 10.3390/molecules28041683. Molecules. 2023. PMID: 36838671 Free PMC article. Review.
References
-
- Nakamura T, et al. Randomized teriparatide [human parathyroid hormone (PTH) 1-34] once-weekly efficacy research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J. Clin. Endocrinol. Metab. 2012;97:3097–3106. doi: 10.1210/jc.2011-3479. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

