New Tmc1 Deafness Mutations Impact Mechanotransduction in Auditory Hair Cells
- PMID: 33824189
- PMCID: PMC8152607
- DOI: 10.1523/JNEUROSCI.2537-20.2021
New Tmc1 Deafness Mutations Impact Mechanotransduction in Auditory Hair Cells
Abstract
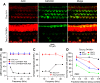
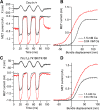
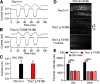
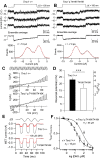
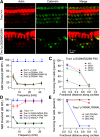
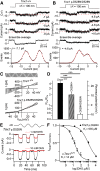
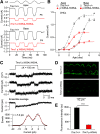
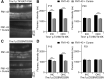
Transmembrane channel-like protein isoform 1 (TMC1) is a major component of the mechano-electrical transducer (MET) channel in cochlear hair cells and is subject to numerous mutations causing deafness. We report a new dominant human deafness mutation, TMC1 p.T422K, and have characterized the homologous mouse mutant, Tmc1 p.T416K, which caused deafness and outer hair cell (OHC) loss by the fourth postnatal week. MET channels showed decreased Ca2+ permeability and resting open probability, but no change in single-channel conductance or expression. Three adjacent deafness mutations are TMC1 p.L416R, p.G417R, and p.M418K, the last homologous to the mouse Beethoven that exhibits similar channel effects. All substitute a positive for a neutral residue, which could produce charge screening in the channel pore or influence binding of an accessory subunit. Channel properties were compared in mice of both sexes between dominant (Tmc1 p.T416K, Tmc1 p.D569N) and recessive (Tmc1 p.W554L, Tmc1 p.D528N) mutations of residues near the putative pore of the channel. Tmc1 p.W554L and p.D569N exhibit reduced maximum current with no effect on single-channel conductance, implying a smaller number of channels transported to the stereociliary tips; this may stem from impaired TMC1 binding to LHFPL5. Tmc1 p.D528N, located in the pore's narrowest region, uniquely caused large reductions in MET channel conductance and block by dihydrostreptomycin (DHS). For Tmc1 p.T416K and Tmc1 p.D528N, transduction loss occurred between P15 and P20. We propose two mechanisms linking channel mutations and deafness: decreased Ca2+ permeability, common to all mutants, and decreased resting open probability in low Ca2+, confined to dominant mutations.SIGNIFICANCE STATEMENT Transmembrane channel-like protein isoform 1 (TMC1) is thought to be a major component of the mechanotransducer channel in auditory hair cells, but the protein organization and channel structure are still uncertain. We made four mouse lines harboring Tmc1 point mutations that alter channel properties, causing hair cell degeneration and deafness. These include a mouse homolog of a new human deafness mutation pT416K that decreased channel Ca2+ permeability by introducing a positively-charged amino acid in the putative pore. All mutations are consistent with the channel structure predicted from modeling, but only one, p.D528N near the external face of the pore, substantially reduced channel conductance and Ca2+ permeability and virtually abolished block by dihydrostreptomycin (DHS), strongly endorsing its siting within the pore.
Keywords: Hair cell; TMC1; cochlea; deafness; mechanotransduction channel.
Copyright © 2021 Beurg et al.
Figures


Similar articles
-
The conductance and organization of the TMC1-containing mechanotransducer channel complex in auditory hair cells.Proc Natl Acad Sci U S A. 2022 Oct 11;119(41):e2210849119. doi: 10.1073/pnas.2210849119. Epub 2022 Oct 3. Proc Natl Acad Sci U S A. 2022. PMID: 36191207 Free PMC article.
-
Tmc1 Point Mutation Affects Ca2+ Sensitivity and Block by Dihydrostreptomycin of the Mechanoelectrical Transducer Current of Mouse Outer Hair Cells.J Neurosci. 2016 Jan 13;36(2):336-49. doi: 10.1523/JNEUROSCI.2439-15.2016. J Neurosci. 2016. PMID: 26758827 Free PMC article.
-
A Tmc1 mutation reduces calcium permeability and expression of mechanoelectrical transduction channels in cochlear hair cells.Proc Natl Acad Sci U S A. 2019 Oct 8;116(41):20743-20749. doi: 10.1073/pnas.1908058116. Epub 2019 Sep 23. Proc Natl Acad Sci U S A. 2019. PMID: 31548403 Free PMC article.
-
Is TMC1 the Hair Cell Mechanotransducer Channel?Biophys J. 2016 Jul 12;111(1):3-9. doi: 10.1016/j.bpj.2016.05.032. Biophys J. 2016. PMID: 27410728 Free PMC article. Review.
-
Mouse tales from Kresge: the deafness mouse.J Am Acad Audiol. 2003 Aug;14(6):296-301. J Am Acad Audiol. 2003. PMID: 14552423 Review.
Cited by
-
LHFPL5 is a key element in force transmission from the tip link to the hair cell mechanotransducer channel.Proc Natl Acad Sci U S A. 2024 Jan 16;121(3):e2318270121. doi: 10.1073/pnas.2318270121. Epub 2024 Jan 9. Proc Natl Acad Sci U S A. 2024. PMID: 38194445 Free PMC article.
-
Mechanoelectrical transduction-related genetic forms of hearing loss.Curr Opin Physiol. 2023 Apr;32:100632. doi: 10.1016/j.cophys.2023.100632. Epub 2023 Jan 25. Curr Opin Physiol. 2023. PMID: 36936795 Free PMC article.
-
Identification of Druggable Binding Sites and Small Molecules as Modulators of TMC1.bioRxiv [Preprint]. 2024 Dec 20:2024.03.05.583611. doi: 10.1101/2024.03.05.583611. bioRxiv. 2024. PMID: 38826329 Free PMC article. Preprint.
-
Regulation of membrane homeostasis by TMC1 mechanoelectrical transduction channels is essential for hearing.Sci Adv. 2022 Aug 5;8(31):eabm5550. doi: 10.1126/sciadv.abm5550. Epub 2022 Aug 3. Sci Adv. 2022. PMID: 35921424 Free PMC article.
-
Novel autosomal dominant TMC1 variants linked to hearing loss: insight into protein-lipid interactions.BMC Med Genomics. 2023 Dec 8;16(1):320. doi: 10.1186/s12920-023-01766-7. BMC Med Genomics. 2023. PMID: 38066485 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
