Effects of fetuin-A-containing calciprotein particles on posttranslational modifications of fetuin-A in HepG2 cells
- PMID: 33820929
- PMCID: PMC8021573
- DOI: 10.1038/s41598-021-86881-0
Effects of fetuin-A-containing calciprotein particles on posttranslational modifications of fetuin-A in HepG2 cells
Abstract
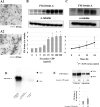
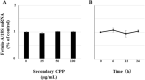
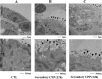
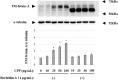
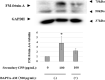
Fetuin-A is an inhibitor of ectopic calcification that is expressed mainly in hepatocytes and is secreted into the circulation after posttranslational processing, including glycosylation and phosphorylation. The molecular weight (MW) of fully modified fetuin-A (FM-fetuin-A) is approximately 60 kDa in an immunoblot, which is much higher than the estimated MW by amino acid sequence. Under conditions of calcification stress such as advanced stage chronic kidney disease, fetuin-A prevents calcification by forming colloidal complexes, which are referred to as calciprotein particles (CPP). Since the significance of CPP in this process is unclear, we investigated the effect of synthetic secondary CPP on the level of FM-fetuin-A in HepG2 cells. Secondary CPP increased the level of FM-fetuin-A in dose- and time-dependent manners, but did not affect expression of mRNA for fetuin-A. Treatment with O- and/or N-glycosidase caused a shift of the 60 kDa band of FM-fetuin-A to a lower MW. Preincubation with brefeldin A, an inhibitor of transport of newly synthesized proteins from the endoplasmic reticulum to the Golgi apparatus, completely blocked the secondary CPP-induced increase in FM-fetuin-A. Treatment with BAPTA-AM, an intracellular calcium chelating agent, also inhibited the CPP-induced increase in the FM-fetuin-A level. Secondary CPP accelerate posttranslational processing of fetuin-A in HepG2 cells.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD.Nephrol Dial Transplant. 2012 May;27(5):1957-66. doi: 10.1093/ndt/gfr609. Epub 2011 Nov 20. Nephrol Dial Transplant. 2012. PMID: 22105144
-
Cellular Clearance and Biological Activity of Calciprotein Particles Depend on Their Maturation State and Crystallinity.Front Immunol. 2018 Sep 4;9:1991. doi: 10.3389/fimmu.2018.01991. eCollection 2018. Front Immunol. 2018. PMID: 30233585 Free PMC article.
-
Post-translational modifications glycosylation and phosphorylation of the major hepatic plasma protein fetuin-A are associated with CNS inflammation in children.PLoS One. 2022 Oct 7;17(10):e0268592. doi: 10.1371/journal.pone.0268592. eCollection 2022. PLoS One. 2022. PMID: 36206263 Free PMC article.
-
Novel assessments of systemic calcification propensity.Curr Opin Nephrol Hypertens. 2016 Jul;25(4):278-84. doi: 10.1097/MNH.0000000000000237. Curr Opin Nephrol Hypertens. 2016. PMID: 27228365 Review.
-
The role of fetuin-A in physiological and pathological mineralization.Calcif Tissue Int. 2013 Oct;93(4):355-64. doi: 10.1007/s00223-012-9690-6. Epub 2013 Jan 1. Calcif Tissue Int. 2013. PMID: 23277412 Review.
Cited by
-
Role of Glycosylation in Vascular Calcification.Int J Mol Sci. 2021 Sep 11;22(18):9829. doi: 10.3390/ijms22189829. Int J Mol Sci. 2021. PMID: 34575990 Free PMC article. Review.
-
Effect of nutritional calcium and phosphate loading on calciprotein particle kinetics in adults with normal and impaired kidney function.Sci Rep. 2022 May 5;12(1):7358. doi: 10.1038/s41598-022-11065-3. Sci Rep. 2022. PMID: 35513558 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

