The Limitless Future of RNA Therapeutics
- PMID: 33816449
- PMCID: PMC8012680
- DOI: 10.3389/fbioe.2021.628137
The Limitless Future of RNA Therapeutics
Abstract
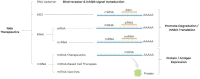
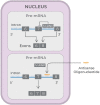
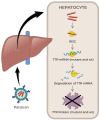
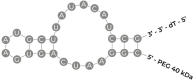
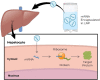
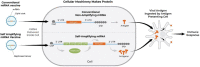
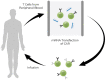
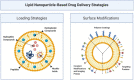
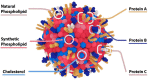
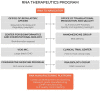
Recent advances in the generation, purification and cellular delivery of RNA have enabled development of RNA-based therapeutics for a broad array of applications. RNA therapeutics comprise a rapidly expanding category of drugs that will change the standard of care for many diseases and actualize personalized medicine. These drugs are cost effective, relatively simple to manufacture, and can target previously undruggable pathways. It is a disruptive therapeutic technology, as small biotech startups, as well as academic groups, can rapidly develop new and personalized RNA constructs. In this review we discuss general concepts of different classes of RNA-based therapeutics, including antisense oligonucleotides, aptamers, small interfering RNAs, microRNAs, and messenger RNA. Furthermore, we provide an overview of the RNA-based therapies that are currently being evaluated in clinical trials or have already received regulatory approval. The challenges and advantages associated with use of RNA-based drugs are also discussed along with various approaches for RNA delivery. In addition, we introduce a new concept of hospital-based RNA therapeutics and share our experience with establishing such a platform at Houston Methodist Hospital.
Keywords: RNA therapeutics; delivery of RNA therapeutics; hospital-based RNA therapeutics; messenger RNAs (mRNAs); self-amplifying mRNA.
Copyright © 2021 Damase, Sukhovershin, Boada, Taraballi, Pettigrew and Cooke.
Conflict of interest statement
Houston Methodist Hospital has been assigned intellectual property related to the synthesis, purification, validation, and delivery of nucleic acid therapeutics. JPC is an inventor on issued patents related to mRNA telomerase therapy, which have been assigned to Stanford University and licensed to his company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
RNA therapeutics: updates and future potential.Sci China Life Sci. 2023 Jan;66(1):12-30. doi: 10.1007/s11427-022-2171-2. Epub 2022 Sep 8. Sci China Life Sci. 2023. PMID: 36100838 Free PMC article. Review.
-
RNA Therapeutics: the Next Generation of Drugs for Cardiovascular Diseases.Curr Atheroscler Rep. 2022 May;24(5):307-321. doi: 10.1007/s11883-022-01007-9. Epub 2022 Apr 2. Curr Atheroscler Rep. 2022. PMID: 35364795 Free PMC article. Review.
-
The infinite possibilities of RNA therapeutics.J Ind Microbiol Biotechnol. 2021 Dec 23;48(9-10):kuab063. doi: 10.1093/jimb/kuab063. J Ind Microbiol Biotechnol. 2021. PMID: 34463324 Free PMC article.
-
Advances in the delivery of RNA therapeutics: from concept to clinical reality.Genome Med. 2017 Jun 27;9(1):60. doi: 10.1186/s13073-017-0450-0. Genome Med. 2017. PMID: 28655327 Free PMC article. Review.
-
The development and technologies of RNA therapeutics.Prog Mol Biol Transl Sci. 2024;203:13-39. doi: 10.1016/bs.pmbts.2023.12.017. Epub 2024 Jan 6. Prog Mol Biol Transl Sci. 2024. PMID: 38359995 Review.
Cited by
-
Unlocking the potential of RNA-based therapeutics in the lung: current status and future directions.Front Genet. 2023 Nov 23;14:1281538. doi: 10.3389/fgene.2023.1281538. eCollection 2023. Front Genet. 2023. PMID: 38075698 Free PMC article. Review.
-
Identification of an Optimal TLR8 Ligand by Alternating the Position of 2'-O-Ribose Methylation.Int J Mol Sci. 2022 Sep 22;23(19):11139. doi: 10.3390/ijms231911139. Int J Mol Sci. 2022. PMID: 36232437 Free PMC article.
-
mRNA Delivery: Challenges and Advances through Polymeric Soft Nanoparticles.Int J Mol Sci. 2024 Feb 1;25(3):1739. doi: 10.3390/ijms25031739. Int J Mol Sci. 2024. PMID: 38339015 Free PMC article. Review.
-
Mitochondrion-targeted RNA therapies as a potential treatment strategy for mitochondrial diseases.Mol Ther Nucleic Acids. 2022 Oct 27;30:359-377. doi: 10.1016/j.omtn.2022.10.012. eCollection 2022 Dec 13. Mol Ther Nucleic Acids. 2022. PMID: 36420220 Free PMC article. Review.
-
Guidelines for mitochondrial RNA analysis.Mol Ther Nucleic Acids. 2024 Jun 26;35(3):102262. doi: 10.1016/j.omtn.2024.102262. eCollection 2024 Sep 10. Mol Ther Nucleic Acids. 2024. PMID: 39091381 Free PMC article. Review.
References
-
- Alnylam (2020a). Alnylam® Development Pipeline of Investigational RNAi Therapeutics Alnylam. Available online at: https://www.alnylam.com/alnylam-rnai-pipeline/ (accessed January 3, 2020).
-
- Alnylam (2020b). Alnylam Announces Approval of GIVLAARITM (givosiran) by the U.S. Food and Drug Administration (FDA) Alnylam Pharm. Inc. Available online at: http://investors.alnylam.com/news-releases/news-release-details/alnylam-... (accessed January 3, 2020).
-
- Amgen (2020). FDA Approves IMLYGIC Talimogene Laherparepvec As First Oncolytic Viral Therapy In The US Amgen Inc. Available online at: http://www.amgen.com/en/media/news-releases/2015/10/fda-approves-imlygic... (accessed August 18, 2020).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

