Iron Accumulation and Lipid Peroxidation in the Aging Retina: Implication of Ferroptosis in Age-Related Macular Degeneration
- PMID: 33815881
- PMCID: PMC7990372
- DOI: 10.14336/AD.2020.0912
Iron Accumulation and Lipid Peroxidation in the Aging Retina: Implication of Ferroptosis in Age-Related Macular Degeneration
Abstract
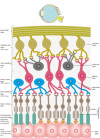
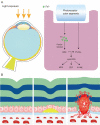
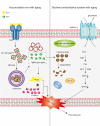
Iron is an essential component in many biological processes in the human body. It is critical for the visual phototransduction cascade in the retina. However, excess iron can be toxic. Iron accumulation and reduced efficiency of intracellular antioxidative defense systems predispose the aging retina to oxidative stress-induced cell death. Age-related macular degeneration (AMD) is characterized by retinal iron accumulation and lipid peroxidation. The mechanisms underlying AMD include oxidative stress-mediated death of retinal pigment epithelium (RPE) cells and subsequent death of retinal photoreceptors. Understanding the mechanism of the disruption of iron and redox homeostasis in the aging retina and AMD is crucial to decipher these mechanisms of cell death and AMD pathogenesis. The mechanisms of retinal cell death in AMD are an area of active investigation; previous studies have proposed several types of cell death as major mechanisms. Ferroptosis, a newly discovered programmed cell death pathway, has been associated with the pathogenesis of several neurodegenerative diseases. Ferroptosis is initiated by lipid peroxidation and is characterized by iron-dependent accumulation. In this review, we provide an overview of the mechanisms of iron accumulation and lipid peroxidation in the aging retina and AMD, with an emphasis on ferroptosis.
Keywords: age-related macular degeneration; ferroptosis; iron; lipid peroxidation; retina.
copyright: © 2021 Zhao et al.
Conflict of interest statement
Conflicts of Interest We declare that we have no conflicts of interest.
Figures

Similar articles
-
Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells.Exp Eye Res. 2019 Apr;181:316-324. doi: 10.1016/j.exer.2018.08.019. Epub 2018 Aug 29. Exp Eye Res. 2019. PMID: 30171859 Free PMC article.
-
Targeting ZIP8 mediated ferroptosis as a novel strategy to protect against the retinal pigment epithelial degeneration.Free Radic Biol Med. 2024 Mar;214:42-53. doi: 10.1016/j.freeradbiomed.2024.01.053. Epub 2024 Feb 1. Free Radic Biol Med. 2024. PMID: 38309537
-
Interferon-γ induces retinal pigment epithelial cell Ferroptosis by a JAK1-2/STAT1/SLC7A11 signaling pathway in Age-related Macular Degeneration.FEBS J. 2022 Apr;289(7):1968-1983. doi: 10.1111/febs.16272. Epub 2021 Nov 22. FEBS J. 2022. PMID: 34741776
-
Ferroptosis as a potential therapeutic target for age-related macular degeneration.Drug Discov Today. 2024 Apr;29(4):103920. doi: 10.1016/j.drudis.2024.103920. Epub 2024 Feb 17. Drug Discov Today. 2024. PMID: 38369100 Review.
-
Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD).Prog Retin Eye Res. 2010 May;29(3):169-90. doi: 10.1016/j.preteyeres.2010.02.002. Epub 2010 Mar 3. Prog Retin Eye Res. 2010. PMID: 20206286 Free PMC article. Review.
Cited by
-
Novel Role of Molecular Hydrogen: The End of Ophthalmic Diseases?Pharmaceuticals (Basel). 2023 Nov 7;16(11):1567. doi: 10.3390/ph16111567. Pharmaceuticals (Basel). 2023. PMID: 38004433 Free PMC article. Review.
-
Establishment of novel ferroptosis-related prognostic subtypes correlating with immune dysfunction in prostate cancer patients.Heliyon. 2023 Dec 9;10(1):e23495. doi: 10.1016/j.heliyon.2023.e23495. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38187257 Free PMC article.
-
Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration.Neural Regen Res. 2023 Jun;18(6):1196-1202. doi: 10.4103/1673-5374.358614. Neural Regen Res. 2023. PMID: 36453394 Free PMC article. Review.
-
Targeting Ferroptosis Holds Potential for Intervertebral Disc Degeneration Therapy.Cells. 2022 Nov 5;11(21):3508. doi: 10.3390/cells11213508. Cells. 2022. PMID: 36359904 Free PMC article. Review.
-
Ferritin But Not Iron Increases in Retina Upon Systemic Iron Overload in Diabetic and Iron-Dextran Injected Mice.Invest Ophthalmol Vis Sci. 2023 Mar 1;64(3):22. doi: 10.1167/iovs.64.3.22. Invest Ophthalmol Vis Sci. 2023. PMID: 36912597 Free PMC article.
References
-
- Hirayama T (2019). Fluorescent probes for the detection of catalytic Fe(II) ion. Free Radic Biol Med, 133:38-45. - PubMed
-
- Masaldan S, Bush AI, Devos D, Rolland AS, Moreau C (2019). Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic Biol Med, 133:221-233. - PubMed
-
- Andrews NC (1999). Disorders of iron metabolism. N Engl J Med, 341:1986-1995. - PubMed
-
- Belaidi AA, Bush AI (2016). Iron neurochemistry in Alzheimer's disease and Parkinson's disease: targets for therapeutics. J Neurochem, 139 Suppl 1:179-197. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
