TRIzol-based RNA extraction for detection protocol for SARS-CoV-2 of coronavirus disease 2019
- PMID: 33815807
- PMCID: PMC8010344
- DOI: 10.1016/j.nmni.2021.100874
TRIzol-based RNA extraction for detection protocol for SARS-CoV-2 of coronavirus disease 2019
Abstract
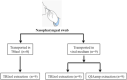
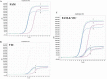
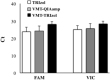
Diagnostic testing is important for managing the 2019 novel coronavirus (SARS-CoV-2). We developed an optimized protocol for SARS-CoV-2 RNA extraction from the surface of the respiratory mucosa with nasopharyngeal swabs and compared the sensitivity of RNA extraction methods. RNA extraction was performed using three different procedures (TRIzol, QIAamp, VMT-TRIzol) from nine positive SARS-CoV-2 samples. SARS-CoV-2 was detected by real-time reverse transcriptase PCR (RT-PCR) using a detection kit for SARS-CoV-2 (Sun Yat-sen University). Compared to RT-PCR results, there were no discernible differences in detection rates when comparing the three different extraction procedures. On the basis of these results, the use of TRIzol as a transport medium and RNA extraction method for SARS-CoV-2 detection may be a helpful alternative for laboratories facing shortages of commercial testing kits.
Keywords: Diagnostic techniques; RT-PCR; SARS-CoV-2; TRIzol; throat swab.
© 2021 Published by Elsevier Ltd.
Figures
Similar articles
-
Automated SARS-COV-2 RNA extraction from patient nasopharyngeal samples using a modified DNA extraction kit for high throughput testing.Ann Saudi Med. 2020 Sep-Oct;40(5):373-381. doi: 10.5144/0256-4947.2020.373. Epub 2020 Oct 1. Ann Saudi Med. 2020. PMID: 32954791 Free PMC article.
-
Detection of SARS-CoV-2 RNA by direct RT-qPCR on nasopharyngeal specimens without extraction of viral RNA.PLoS One. 2020 Jul 24;15(7):e0236564. doi: 10.1371/journal.pone.0236564. eCollection 2020. PLoS One. 2020. PMID: 32706827 Free PMC article.
-
Alternative RNA extraction-free techniques for the real-time RT-PCR detection of SARS-CoV-2 in nasopharyngeal swab and sputum samples.J Virol Methods. 2021 Dec;298:114302. doi: 10.1016/j.jviromet.2021.114302. Epub 2021 Sep 23. J Virol Methods. 2021. PMID: 34563582 Free PMC article.
-
SARS-CoV-2: Comparative analysis of different RNA extraction methods.J Virol Methods. 2021 Jan;287:114008. doi: 10.1016/j.jviromet.2020.114008. Epub 2020 Nov 4. J Virol Methods. 2021. PMID: 33160015 Free PMC article.
-
Evaluation of simple nucleic acid extraction methods for the detection of SARS-CoV-2 in nasopharyngeal and saliva specimens during global shortage of extraction kits.J Clin Virol. 2020 Aug;129:104519. doi: 10.1016/j.jcv.2020.104519. Epub 2020 Jun 23. J Clin Virol. 2020. PMID: 32629187 Free PMC article.
Cited by
-
MFAP2 induces epithelial-mesenchymal transformation of osteosarcoma cells by activating the Notch1 pathway.Transl Cancer Res. 2024 Jun 30;13(6):2847-2859. doi: 10.21037/tcr-23-2035. Epub 2024 Jun 27. Transl Cancer Res. 2024. PMID: 38988940 Free PMC article.
-
Low temperature upregulating HSP70 expression to mitigate the paclitaxel-induced damages in NHEK cell.PeerJ. 2023 Jan 17;11:e14630. doi: 10.7717/peerj.14630. eCollection 2023. PeerJ. 2023. PMID: 36684674 Free PMC article.
-
MOLECULAR DETECTION PROTOCOL OF SARS-COV-2 THROUGH SELF-COLLECTED SALIVA SPECIMENS VERSUS NASOPHARYNGEAL SWABS.Afr J Infect Dis. 2024 Mar 8;18(2):1-7. doi: 10.21010/Ajidv18i2.1. eCollection 2024. Afr J Infect Dis. 2024. PMID: 38606190 Free PMC article.
-
Campus source to sink wastewater surveillance of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).Curr Res Microb Sci. 2024 May 6;6:100240. doi: 10.1016/j.crmicr.2024.100240. eCollection 2024. Curr Res Microb Sci. 2024. PMID: 38774836 Free PMC article.
-
An inexpensive and rapid diagnostic method for detection of SARS-CoV-2 RNA by loop-mediated isothermal amplification (LAMP).MethodsX. 2023;10:102011. doi: 10.1016/j.mex.2023.102011. Epub 2023 Jan 11. MethodsX. 2023. PMID: 36643803 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
