Clinical outcomes after permanent polymer or polymer-free stent implantation in patients with diabetes mellitus: The ReCre8 diabetes substudy
- PMID: 33811730
- PMCID: PMC9540458
- DOI: 10.1002/ccd.29685
Clinical outcomes after permanent polymer or polymer-free stent implantation in patients with diabetes mellitus: The ReCre8 diabetes substudy
Abstract
Objectives: The purpose of this analysis was to compare target-lesion failure (TLF) of a permanent polymer zotarolimus-eluting stent (PP-ZES) versus a polymer-free amphilimus-eluting stent (PF-AES) in diabetics.
Background: The improvement of outcomes with new-generation drug-eluting stent as seen in the general population is less pronounced among diabetics. The PF-AES introduces an elution-technology with potential enhanced performance in diabetics.
Methods: In this subanalysis of the ReCre8 trial, patients were randomized to either a PP-ZES or PF-AES after stratification for diabetes and troponin status. The primary device-oriented endpoint was TLF, a composite of cardiac death, target-vessel myocardial infarction and target-lesion revascularization.
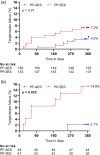
Results: In the ReCre8 trial, 304 (20%) patients were diabetic and 96 (6%) had insulin-dependent diabetes mellitus. There was no statistically significant difference between the two study arms regarding the primary endpoint (PP-ZES 7.2% vs. PF-AES 4.0%; p = .21), although the composite of net adverse clinical events was higher in the PP-ZES arm (15.7 vs. 8.0%; p = .035). Stent thrombosis was low in both groups with no cases in the PP-ZES arm and 1 case in the PF-AES arm (p = .32). Regarding insulin-treated diabetics, TLF was higher in the PP-ZES arm (14.9 vs. 2.1%; p = .022).
Conclusions: Diabetics could potentially benefit from a dedicated stent, releasing sirolimus with a lipophilic carrier (amphilimus-formulation). Future trials should confirm the potential benefit of a PF-AES in this population.
Keywords: diabetes mellitus; drug-eluting stents; percutaneous coronary intervention.
© 2021 The Authors. Catheterization and Cardiovascular Interventions published by Wiley Periodicals LLC.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Long-term follow-up of contemporary drug-eluting stent implantation in diabetic patients: Subanalysis of a randomized controlled trial.Catheter Cardiovasc Interv. 2023 Feb;101(3):505-510. doi: 10.1002/ccd.30545. Epub 2023 Jan 18. Catheter Cardiovasc Interv. 2023. PMID: 36651339 Clinical Trial.
-
Randomized All-Comers Evaluation of a Permanent Polymer Zotarolimus-Eluting Stent Versus a Polymer-Free Amphilimus-Eluting Stent.Circulation. 2019 Jan 2;139(1):67-77. doi: 10.1161/CIRCULATIONAHA.118.037707. Circulation. 2019. PMID: 30586704 Clinical Trial.
-
3-Year Clinical Outcomes After Implantation of Permanent-Polymer Versus Polymer-Free Stent: ReCre8 Landmark Analysis.JACC Cardiovasc Interv. 2021 Nov 22;14(22):2477-2486. doi: 10.1016/j.jcin.2021.08.078. JACC Cardiovasc Interv. 2021. PMID: 34794654 Clinical Trial.
-
One-year clinical outcomes of patients treated with polymer-free amphilimus-eluting stents or zotarolimus-eluting stents: A propensity-score adjusted analysis.Catheter Cardiovasc Interv. 2019 Jul 1;94(1):61-69. doi: 10.1002/ccd.28041. Epub 2019 Jan 2. Catheter Cardiovasc Interv. 2019. PMID: 30604493 Free PMC article.
-
Ten-year clinical outcomes of polymer-free versus durable polymer new-generation drug-eluting stent in patients with coronary artery disease with and without diabetes mellitus : Results of the Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus- and Probucol- and Zotarolimus-Eluting Stents (ISAR-TEST 5) trial.Clin Res Cardiol. 2021 Oct;110(10):1586-1598. doi: 10.1007/s00392-021-01854-7. Epub 2021 Jun 22. Clin Res Cardiol. 2021. PMID: 34156521 Free PMC article. Clinical Trial.
Cited by
-
Polymer-free stents for percutaneous coronary intervention in diabetic patients: a systematic review and meta-analysis.Future Cardiol. 2024;20(9):485-497. doi: 10.1080/14796678.2024.2370688. Epub 2024 Jul 9. Future Cardiol. 2024. PMID: 38980301
-
Amphilimus- vs. zotarolimus-eluting stents in patients with diabetes mellitus and coronary artery disease: the SUGAR trial.Eur Heart J. 2022 Mar 31;43(13):1320-1330. doi: 10.1093/eurheartj/ehab790. Eur Heart J. 2022. PMID: 34735004 Free PMC article. Clinical Trial.
-
One-year outcomes of polymer-free amphilimus-eluting stents versus durable polymer zotarolimus-eluting stents in patients with diabetes mellitus: a meta-analysis.Cardiovasc Diabetol. 2022 Oct 28;21(1):220. doi: 10.1186/s12933-022-01673-8. Cardiovasc Diabetol. 2022. PMID: 36307791 Free PMC article.
References
-
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570‐2581. - PubMed
-
- Richardt G, Maillard L, Nazzaro MS, et al. Polymer‐free drug‐coated coronary stents in diabetic patients at high bleeding risk: a pre‐specified sub‐study of the LEADERS FREE trial. Clin Res Cardiol. 2019;108:31‐38. - PubMed
-
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40‐50. - PubMed
-
- Lee TT, Feinberg L, Baim DS, et al. Effect of diabetes mellitus on five‐year clinical outcomes after single‐vessel coronary stenting (a pooled analysis of coronary stent clinical trials). Am J Cardiol. 2006;98:718‐721. - PubMed
-
- Kalkman DN, Woudstra P, den Heijer P, et al. One year clinical outcomes in patients with insulin‐treated diabetes mellitus and non‐insulin‐treated diabetes mellitus compared to non‐diabetics after deployment of the bio‐engineered COMBO stent. Int J Cardiol. 2017;226:60‐64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

