The Inflammatory Profile of Obesity and the Role on Pulmonary Bacterial and Viral Infections
- PMID: 33810619
- PMCID: PMC8037155
- DOI: 10.3390/ijms22073456
The Inflammatory Profile of Obesity and the Role on Pulmonary Bacterial and Viral Infections
Abstract
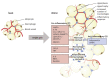
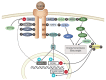
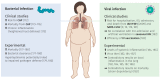
Obesity is a globally increasing health problem, entailing diverse comorbidities such as infectious diseases. An obese weight status has marked effects on lung function that can be attributed to mechanical dysfunctions. Moreover, the alterations of adipocyte-derived signal mediators strongly influence the regulation of inflammation, resulting in chronic low-grade inflammation. Our review summarizes the known effects regarding pulmonary bacterial and viral infections. For this, we discuss model systems that allow mechanistic investigation of the interplay between obesity and lung infections. Overall, obesity gives rise to a higher susceptibility to infectious pathogens, but the pathogenetic process is not clearly defined. Whereas, viral infections often show a more severe course in obese patients, the same patients seem to have a survival benefit during bacterial infections. In particular, we summarize the main mechanical impairments in the pulmonary tract caused by obesity. Moreover, we outline the main secretory changes within the expanded adipose tissue mass, resulting in chronic low-grade inflammation. Finally, we connect these altered host factors to the influence of obesity on the development of lung infection by summarizing observations from clinical and experimental data.
Keywords: adipocytokines; bacteria; lung infection; obesity; obesity paradox; viruses.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Obesity and lung inflammation.J Appl Physiol (1985). 2010 Mar;108(3):722-8. doi: 10.1152/japplphysiol.00781.2009. Epub 2009 Oct 29. J Appl Physiol (1985). 2010. PMID: 19875709 Free PMC article. Review.
-
Cellular hypoxia and adipose tissue dysfunction in obesity.Proc Nutr Soc. 2009 Nov;68(4):370-7. doi: 10.1017/S0029665109990206. Epub 2009 Aug 24. Proc Nutr Soc. 2009. PMID: 19698203 Review.
-
A Perfect Storm: Increased Colonization and Failure of Vaccination Leads to Severe Secondary Bacterial Infection in Influenza Virus-Infected Obese Mice.mBio. 2017 Sep 19;8(5):e00889-17. doi: 10.1128/mBio.00889-17. mBio. 2017. PMID: 28928207 Free PMC article.
-
Obesity, adipokines, and lung disease.J Appl Physiol (1985). 2010 Mar;108(3):744-53. doi: 10.1152/japplphysiol.00838.2009. Epub 2009 Nov 19. J Appl Physiol (1985). 2010. PMID: 19926824 Free PMC article. Review.
-
Vitamin B2 deficiency enhances the pro-inflammatory activity of adipocyte, consequences for insulin resistance and metabolic syndrome development.Life Sci. 2017 Jun 1;178:9-16. doi: 10.1016/j.lfs.2017.04.010. Epub 2017 Apr 14. Life Sci. 2017. PMID: 28414075
Cited by
-
Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity.Int J Mol Sci. 2022 Jul 1;23(13):7349. doi: 10.3390/ijms23137349. Int J Mol Sci. 2022. PMID: 35806353 Free PMC article. Review.
-
The negative association between weight-adjusted-waist index and lung functions: NHANES 2007-2012.PLoS One. 2024 Oct 23;19(10):e0311619. doi: 10.1371/journal.pone.0311619. eCollection 2024. PLoS One. 2024. PMID: 39441792 Free PMC article.
-
Fatty Acids as Potent Modulators of Autophagy Activity in White Adipose Tissue.Biomolecules. 2023 Jan 30;13(2):255. doi: 10.3390/biom13020255. Biomolecules. 2023. PMID: 36830623 Free PMC article. Review.
-
Cross-Talk of NADPH Oxidases and Inflammation in Obesity.Antioxidants (Basel). 2023 Aug 9;12(8):1589. doi: 10.3390/antiox12081589. Antioxidants (Basel). 2023. PMID: 37627585 Free PMC article. Review.
-
The Immune Response in Adipocytes and Their Susceptibility to Infection: A Possible Relationship with Infectobesity.Int J Mol Sci. 2022 May 31;23(11):6154. doi: 10.3390/ijms23116154. Int J Mol Sci. 2022. PMID: 35682832 Free PMC article. Review.
References
-
- Rosalki S.B. Scientific Foundations of Biochemistry in Clinical Practice. Elsevier; Amsterdam, The Netherlands: 1994. The Clinical Biochemistry of Alcohol; pp. 121–143. - DOI
-
- Obesity and Overweight. [(accessed on 7 November 2020)]; Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

