Integration of Data from Liquid-Liquid Phase Separation Databases Highlights Concentration and Dosage Sensitivity of LLPS Drivers
- PMID: 33809541
- PMCID: PMC8002189
- DOI: 10.3390/ijms22063017
Integration of Data from Liquid-Liquid Phase Separation Databases Highlights Concentration and Dosage Sensitivity of LLPS Drivers
Abstract
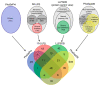
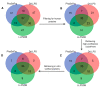
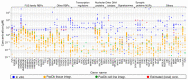
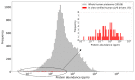
Liquid-liquid phase separation (LLPS) is a molecular process that leads to the formation of membraneless organelles, representing functionally specialized liquid-like cellular condensates formed by proteins and nucleic acids. Integrating the data on LLPS-associated proteins from dedicated databases revealed only modest agreement between them and yielded a high-confidence dataset of 89 human LLPS drivers. Analysis of the supporting evidence for our dataset uncovered a systematic and potentially concerning difference between protein concentrations used in a good fraction of the in vitro LLPS experiments, a key parameter that governs the phase behavior, and the proteomics-derived cellular abundance levels of the corresponding proteins. Closer scrutiny of the underlying experimental data enabled us to offer a sound rationale for this systematic difference, which draws on our current understanding of the cellular organization of the proteome and the LLPS process. In support of this rationale, we find that genes coding for our human LLPS drivers tend to be dosage-sensitive, suggesting that their cellular availability is tightly regulated to preserve their functional role in direct or indirect relation to condensate formation. Our analysis offers guideposts for increasing agreement between in vitro and in vivo studies, probing the roles of proteins in LLPS.
Keywords: data integration; dosage sensitivity; liquid demixing; liquid–liquid phase separation; local concentration; membraneless organelles; protein abundance; quantitative proteomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Computational resources for identifying and describing proteins driving liquid-liquid phase separation.Brief Bioinform. 2021 Sep 2;22(5):bbaa408. doi: 10.1093/bib/bbaa408. Brief Bioinform. 2021. PMID: 33517364 Free PMC article.
-
DrLLPS: a data resource of liquid-liquid phase separation in eukaryotes.Nucleic Acids Res. 2020 Jan 8;48(D1):D288-D295. doi: 10.1093/nar/gkz1027. Nucleic Acids Res. 2020. PMID: 31691822 Free PMC article.
-
Phase Separation of Epstein-Barr Virus EBNA2 and Its Coactivator EBNALP Controls Gene Expression.J Virol. 2020 Mar 17;94(7):e01771-19. doi: 10.1128/JVI.01771-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31941785 Free PMC article.
-
Liquid-liquid phase separation (LLPS) in cellular physiology and tumor biology.Am J Cancer Res. 2021 Aug 15;11(8):3766-3776. eCollection 2021. Am J Cancer Res. 2021. PMID: 34522448 Free PMC article. Review.
-
Liquid-Liquid Phase Separation in Crowded Environments.Int J Mol Sci. 2020 Aug 17;21(16):5908. doi: 10.3390/ijms21165908. Int J Mol Sci. 2020. PMID: 32824618 Free PMC article. Review.
Cited by
-
VUS next in rare diseases? Deciphering genetic determinants of biomolecular condensation.Orphanet J Rare Dis. 2024 Sep 6;19(1):327. doi: 10.1186/s13023-024-03307-6. Orphanet J Rare Dis. 2024. PMID: 39243101 Free PMC article. Review.
-
The Role of Intrinsically Disordered Proteins in Liquid-Liquid Phase Separation during Calcium Carbonate Biomineralization.Biomolecules. 2022 Sep 9;12(9):1266. doi: 10.3390/biom12091266. Biomolecules. 2022. PMID: 36139105 Free PMC article. Review.
-
A sePARate phase? Poly(ADP-ribose) versus RNA in the organization of biomolecular condensates.Nucleic Acids Res. 2022 Oct 28;50(19):10817-10838. doi: 10.1093/nar/gkac866. Nucleic Acids Res. 2022. PMID: 36243979 Free PMC article. Review.
-
Biomolecular condensate phase diagrams with a combinatorial microdroplet platform.Nat Commun. 2022 Dec 21;13(1):7845. doi: 10.1038/s41467-022-35265-7. Nat Commun. 2022. PMID: 36543777 Free PMC article.
-
Evolutionary Study of Protein Short Tandem Repeats in Protein Families.Biomolecules. 2023 Jul 13;13(7):1116. doi: 10.3390/biom13071116. Biomolecules. 2023. PMID: 37509152 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

