Comparisons of Extracellular Vesicles from Human Epidural Fat-Derived Mesenchymal Stem Cells and Fibroblast Cells
- PMID: 33809214
- PMCID: PMC8000612
- DOI: 10.3390/ijms22062889
Comparisons of Extracellular Vesicles from Human Epidural Fat-Derived Mesenchymal Stem Cells and Fibroblast Cells
Abstract
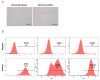
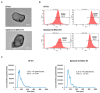
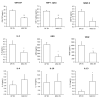
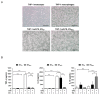
Extracellular vesicles (EVs) are generated and secreted by cells into the circulatory system. Stem cell-derived EVs have a therapeutic effect similar to that of stem cells and are considered an alternative method for cell therapy. Accordingly, research on the characteristics of EVs is emerging. EVs were isolated from human epidural fat-derived mesenchymal stem cells (MSCs) and human fibroblast culture media by ultracentrifugation. The characterization of EVs involved the typical evaluation of cluster of differentiation (CD antigens) marker expression by fluorescence-activated cell sorting, size analysis with dynamic laser scattering, and morphology analysis with transmission electron microscopy. Lastly, the secreted levels of cytokines and chemokines in EVs were determined by a cytokine assay. The isolated EVs had a typical size of approximately 30-200 nm, and the surface proteins CD9 and CD81 were expressed on human epidural fat MSCs and human fibroblast cells. The secreted levels of cytokines and chemokines were compared between human epidural fat MSC-derived EVs and human fibroblast-derived EVs. Human epidural fat MSC-derived EVs showed anti-inflammatory effects and promoted macrophage polarization. In this study, we demonstrated for the first time that human epidural fat MSC-derived EVs exhibit inflammatory suppressive potency relative to human fibroblast-derived EVs, which may be useful for the treatment of inflammation-related diseases.
Keywords: chemokine; cytokine; epidural fat; extracellular vesicle; fibroblast; inflammation; mesenchymal stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content.J Cell Physiol. 2019 Jun;234(6):8249-8258. doi: 10.1002/jcp.27669. Epub 2018 Oct 30. J Cell Physiol. 2019. PMID: 30378105 Review.
-
Monocyte-derived extracellular vesicles stimulate cytokine secretion and gene expression of matrix metalloproteinases by mesenchymal stem/stromal cells.FEBS J. 2018 Jun;285(12):2337-2359. doi: 10.1111/febs.14485. Epub 2018 May 18. FEBS J. 2018. PMID: 29732732
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization.Stem Cells Transl Med. 2017 Mar;6(3):1018-1028. doi: 10.1002/sctm.16-0363. Epub 2017 Jan 31. Stem Cells Transl Med. 2017. PMID: 28186708 Free PMC article.
-
Cellular In Vitro Responses Induced by Human Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles Obtained from Suspension Culture.Int J Mol Sci. 2024 Jul 11;25(14):7605. doi: 10.3390/ijms25147605. Int J Mol Sci. 2024. PMID: 39062847 Free PMC article.
-
Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases.Cells. 2019 Dec 11;8(12):1605. doi: 10.3390/cells8121605. Cells. 2019. PMID: 31835680 Free PMC article. Review.
Cited by
-
The Evolving Landscape of Potency Assays.Adv Exp Med Biol. 2023;1420:165-189. doi: 10.1007/978-3-031-30040-0_11. Adv Exp Med Biol. 2023. PMID: 37258790
-
Serum-Derived Neuronal Exosomal miRNAs as Biomarkers of Acute Severe Stress.Int J Mol Sci. 2021 Sep 15;22(18):9960. doi: 10.3390/ijms22189960. Int J Mol Sci. 2021. PMID: 34576126 Free PMC article.
-
Proteomic profiling of extracellular vesicles derived from human serum for the discovery of biomarkers in Avascular necrosis.Clin Proteomics. 2024 Jun 2;21(1):39. doi: 10.1186/s12014-024-09489-2. Clin Proteomics. 2024. PMID: 38825675 Free PMC article.
-
Extracellular Vesicle Derived From Mesenchymal Stem Cells Have Bidirectional Effects on the Development of Lung Cancer.Front Oncol. 2022 Jul 4;12:914832. doi: 10.3389/fonc.2022.914832. eCollection 2022. Front Oncol. 2022. PMID: 35860555 Free PMC article. Review.
-
Human Epidural AD-MSC Exosomes Improve Function Recovery after Spinal Cord Injury in Rats.Biomedicines. 2022 Mar 15;10(3):678. doi: 10.3390/biomedicines10030678. Biomedicines. 2022. PMID: 35327480 Free PMC article.
References
-
- Greening D.W., Xu R., Ji H., Tauro B.J., Simpson R.J. A Protocol for Exosome Isolation and Characterization: Evaluation of Ultracentrifugation, Density-Gradient Separation, and Immunoaffinity Capture Methods. Methods Mol. Biol. 2015;1295:179–209. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

