Recent Advances in the Molecular Design and Applications of Multispecific Biotherapeutics
- PMID: 33808165
- PMCID: PMC8103270
- DOI: 10.3390/antib10020013
Recent Advances in the Molecular Design and Applications of Multispecific Biotherapeutics
Abstract
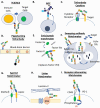
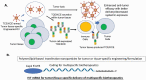
Recombinant protein-based biotherapeutics drugs have transformed clinical pipelines of the biopharmaceutical industry since the launch of recombinant insulin nearly four decades ago. These biologic drugs are structurally more complex than small molecules, and yet share a similar principle for rational drug discovery and development: That is to start with a pre-defined target and follow with the functional modulation with a therapeutic agent. Despite these tremendous successes, this "one target one drug" paradigm has been challenged by complex disease mechanisms that involve multiple pathways and demand new therapeutic routes. A rapidly evolving wave of multispecific biotherapeutics is coming into focus. These new therapeutic drugs are able to engage two or more protein targets via distinct binding interfaces with or without the chemical conjugation to large or small molecules. They possess the potential to not only address disease intricacy but also exploit new therapeutic mechanisms and assess undruggable targets for conventional monospecific biologics. This review focuses on the recent advances in molecular design and applications of major classes of multispecific biotherapeutics drugs, which include immune cells engagers, antibody-drug conjugates, multispecific tetherbodies, biologic matchmakers, and small-scaffold multispecific modalities. Challenges posed by the multispecific biotherapeutics drugs and their future outlooks are also discussed.
Keywords: antibody-drug conjugates; biologic matchmakers; immune cells engagers; multispecific biotherapeutics; multispecific tetherbodies; small-scaffold multispecific modalities.
Conflict of interest statement
X.Z. and A.M.D. currently are employees of Pfizer Worldwide Research, Cambridge, MA.
Figures
Similar articles
-
Multispecific drugs herald a new era of biopharmaceutical innovation.Nature. 2020 Apr;580(7803):329-338. doi: 10.1038/s41586-020-2168-1. Epub 2020 Apr 15. Nature. 2020. PMID: 32296187 Review.
-
Mechanism-Driven Design of Multispecific Antibodies for Targeted Disease Treatment.Annu Rev Chem Biomol Eng. 2024 Jan 26. doi: 10.1146/annurev-chembioeng-100522-102155. Online ahead of print. Annu Rev Chem Biomol Eng. 2024. PMID: 38277673 Review.
-
How can we discover developable antibody-based biotherapeutics?Front Mol Biosci. 2023 Aug 7;10:1221626. doi: 10.3389/fmolb.2023.1221626. eCollection 2023. Front Mol Biosci. 2023. PMID: 37609373 Free PMC article. Review.
-
Molecular imaging supports the development of multispecific cancer antibodies.Nat Rev Clin Oncol. 2024 Dec;21(12):852-866. doi: 10.1038/s41571-024-00946-3. Epub 2024 Sep 26. Nat Rev Clin Oncol. 2024. PMID: 39327536 Review.
-
Rationale and development of multispecific antibody drugs.Expert Rev Clin Pharmacol. 2010 Jul;3(4):491-508. doi: 10.1586/ecp.10.28. Expert Rev Clin Pharmacol. 2010. PMID: 22111679
Cited by
-
Identification of Mispairing Omic Signatures in Chinese Hamster Ovary (CHO) Cells Producing a Tri-Specific Antibody.Biomedicines. 2023 Oct 25;11(11):2890. doi: 10.3390/biomedicines11112890. Biomedicines. 2023. PMID: 38001891 Free PMC article.
-
Embedding Dynamics in Intrinsic Physicochemical Profiles of Market-Stage Antibody-Based Biotherapeutics.Mol Pharm. 2023 Feb 6;20(2):1096-1111. doi: 10.1021/acs.molpharmaceut.2c00838. Epub 2022 Dec 27. Mol Pharm. 2023. PMID: 36573887 Free PMC article.
-
A potential antibody repertoire diversification mechanism through tyrosine sulfation for biotherapeutics engineering and production.Front Immunol. 2022 Dec 8;13:1072702. doi: 10.3389/fimmu.2022.1072702. eCollection 2022. Front Immunol. 2022. PMID: 36569848 Free PMC article.
-
High-Resolution Demultiplexing (HRdm) Ion Mobility Spectrometry-Mass Spectrometry for Aspartic and Isoaspartic Acid Determination and Screening.Anal Chem. 2022 Apr 26;94(16):6191-6199. doi: 10.1021/acs.analchem.1c05533. Epub 2022 Apr 14. Anal Chem. 2022. PMID: 35421308 Free PMC article.
-
Next generation of multispecific antibody engineering.Antib Ther. 2023 Dec 8;7(1):37-52. doi: 10.1093/abt/tbad027. eCollection 2024 Jan. Antib Ther. 2023. PMID: 38235376 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

