Probing Skin Barrier Recovery on Molecular Level Following Acute Wounds: An In Vivo/Ex Vivo Study on Pigs
- PMID: 33807251
- PMCID: PMC8065685
- DOI: 10.3390/biomedicines9040360
Probing Skin Barrier Recovery on Molecular Level Following Acute Wounds: An In Vivo/Ex Vivo Study on Pigs
Abstract
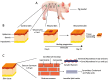
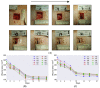
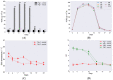
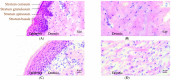
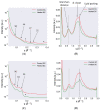
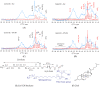
Proper skin barrier function is paramount for our survival, and, suffering injury, there is an acute need to restore the lost barrier and prevent development of a chronic wound. We hypothesize that rapid wound closure is more important than immediate perfection of the barrier, whereas specific treatment may facilitate perfection. The aim of the current project was therefore to evaluate the quality of restored tissue down to the molecular level. We used Göttingen minipigs with a multi-technique approach correlating wound healing progression in vivo over three weeks, monitored by classical methods (e.g., histology, trans-epidermal water loss (TEWL), pH) and subsequent physicochemical characterization of barrier recovery (i.e., small and wide-angle X-ray diffraction (SWAXD), polarization transfer solid-state NMR (PTssNMR), dynamic vapor sorption (DVS), Fourier transform infrared (FTIR)), providing a unique insight into molecular aspects of healing. We conclude that although acute wounds sealed within two weeks as expected, molecular investigation of stratum corneum (SC) revealed a poorly developed keratin organization and deviations in lipid lamellae formation. A higher lipid fluidity was also observed in regenerated tissue. This may have been due to incomplete lipid conversion during barrier recovery as glycosphingolipids, normally not present in SC, were indicated by infrared FTIR spectroscopy. Evidently, a molecular approach to skin barrier recovery could be a valuable tool in future development of products targeting wound healing.
Keywords: acute wound; histology; in vivo/ex vivo; lipid; pH; polarization transfer solid state NMR (PTssNMR); skin barrier; small and wide-angle X-ray diffraction (SWAXD); stratum corneum; trans-epidermal water loss (TEWL).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Unraveling UVB effects: Catalase activity and molecular alterations in the stratum corneum.J Colloid Interface Sci. 2024 Jul 15;666:176-188. doi: 10.1016/j.jcis.2024.03.200. Epub 2024 Mar 30. J Colloid Interface Sci. 2024. PMID: 38593652
-
In vivo barrier challenge and long-term recovery in human facial skin.Int J Cosmet Sci. 2013 Jun;35(3):250-6. doi: 10.1111/ics.12034. Epub 2013 Jan 24. Int J Cosmet Sci. 2013. PMID: 23278506
-
Facial skin pigmentation is not related to stratum corneum cohesion, basal transepidermal water loss, barrier integrity and barrier repair.Int J Cosmet Sci. 2015 Apr;37(2):241-52. doi: 10.1111/ics.12189. Epub 2015 Jan 21. Int J Cosmet Sci. 2015. PMID: 25482263
-
Update of technologies for examining the stratum corneum at the molecular level.Br J Dermatol. 2014 Sep;171 Suppl 3:13-8. doi: 10.1111/bjd.13280. Br J Dermatol. 2014. PMID: 25234173 Review.
-
Barrier function of the skin: "la raison d'être" of the epidermis.J Invest Dermatol. 2003 Aug;121(2):231-41. doi: 10.1046/j.1523-1747.2003.12359.x. J Invest Dermatol. 2003. PMID: 12880413 Review.
Cited by
-
Dermal Absorption of Sesquiterpene Lactones from Arnica Tincture.Pharmaceutics. 2022 Mar 29;14(4):742. doi: 10.3390/pharmaceutics14040742. Pharmaceutics. 2022. PMID: 35456576 Free PMC article.
References
-
- Schaefer H., Redelmeier T.E. Skin Barrier: Principles of Percutaneous Absorption. Karger; Basel, Switzerland: 1996.
-
- Scheuplein R.J. Permeability of the skin: A review of major concepts and some new developments. J. Investig. Dermatol. 1976;67:672–676. doi: 10.1111/1523-1747.ep12544513. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

