Delivery of Orally Administered Digestible Antibodies Using Nanoparticles
- PMID: 33805888
- PMCID: PMC8036930
- DOI: 10.3390/ijms22073349
Delivery of Orally Administered Digestible Antibodies Using Nanoparticles
Abstract
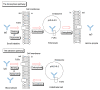
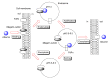
Oral administration of medications is highly preferred in healthcare owing to its simplicity and convenience; however, problems of drug membrane permeability can arise with any administration method in drug discovery and development. In particular, commonly used monoclonal antibody (mAb) drugs are directly injected through intravenous or subcutaneous routes across physical barriers such as the cell membrane, including the epithelium and endothelium. However, intravenous administration has disadvantages such as pain, discomfort, and stress. Oral administration is an ideal route for mAbs. Nonetheless, proteolysis and denaturation, in addition to membrane impermeability, pose serious challenges in delivering peroral mAbs to the systemic circulation, biologically, through enzymatic and acidic blocks and, physically, through the small intestinal epithelium barrier. A number of clinical trials have been performed using oral mAbs for the local treatment of gastrointestinal diseases, some of which have adopted capsules or tablets as formulations. Surprisingly, no oral mAbs have been approved clinically. An enteric nanodelivery system can protect cargos from proteolysis and denaturation. Moreover, mAb cargos released in the small intestine may be delivered to the systemic circulation across the intestinal epithelium through receptor-mediated transcytosis. Oral Abs in milk are transported by neonatal Fc receptors to the systemic circulation in neonates. Thus, well-designed approaches can establish oral mAb delivery. In this review, I will introduce the implementation and possibility of delivering orally administered mAbs with or without nanoparticles not only to the local gastrointestinal tract but also to the systemic circulation.
Keywords: drug delivery system; mouth-to-systemic circulation monoclonal antibody delivery; nanodelivery; neonatal Fc receptor-mediated transcytosis; oral immunotherapy; orally administered monoclonal antibodies.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Shortcut Approaches to Substance Delivery into the Brain Based on Intranasal Administration Using Nanodelivery Strategies for Insulin.Molecules. 2020 Nov 7;25(21):5188. doi: 10.3390/molecules25215188. Molecules. 2020. PMID: 33171799 Free PMC article. Review.
-
Transepithelial transport of Fc-targeted nanoparticles by the neonatal fc receptor for oral delivery.Sci Transl Med. 2013 Nov 27;5(213):213ra167. doi: 10.1126/scitranslmed.3007049. Sci Transl Med. 2013. PMID: 24285486 Free PMC article.
-
The biological challenges and pharmacological opportunities of orally administered nanomedicine delivery.Expert Rev Gastroenterol Hepatol. 2018 Mar;12(3):223-236. doi: 10.1080/17474124.2018.1399794. Epub 2017 Nov 8. Expert Rev Gastroenterol Hepatol. 2018. PMID: 29088978 Review.
-
Cellular uptake and transcytosis of lipid-based nanoparticles across the intestinal barrier: Relevance for oral drug delivery.J Colloid Interface Sci. 2016 Feb 1;463:258-65. doi: 10.1016/j.jcis.2015.10.057. Epub 2015 Oct 24. J Colloid Interface Sci. 2016. PMID: 26550783
-
Targeted localized use of therapeutic antibodies: a review of non-systemic, topical and oral applications.Crit Rev Biotechnol. 2016;36(3):506-20. doi: 10.3109/07388551.2014.992388. Epub 2015 Jan 20. Crit Rev Biotechnol. 2016. PMID: 25600465
Cited by
-
Therapeutic antibodies - natural and pathological barriers and strategies to overcome them.Pharmacol Ther. 2022 May;233:108022. doi: 10.1016/j.pharmthera.2021.108022. Epub 2021 Oct 20. Pharmacol Ther. 2022. PMID: 34687769 Free PMC article. Review.
-
Progress of polymer-based strategies in fungal disease management: Designed for different roles.Front Cell Infect Microbiol. 2023 Mar 22;13:1142029. doi: 10.3389/fcimb.2023.1142029. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37033476 Free PMC article. Review.
-
Feasibility of Polyclonal Avian Immunoglobulins (IgY) as Prophylaxis against Human Norovirus Infection.Viruses. 2022 Oct 27;14(11):2371. doi: 10.3390/v14112371. Viruses. 2022. PMID: 36366469 Free PMC article.
-
Mechanism-driven strategies for prevention of rheumatoid arthritis.Rheumatol Autoimmun. 2022 Sep;2(3):109-119. doi: 10.1002/rai2.12043. Epub 2022 Jun 15. Rheumatol Autoimmun. 2022. PMID: 36312783 Free PMC article.
-
Brain Cancer Chemotherapy through a Delivery System across the Blood-Brain Barrier into the Brain Based on Receptor-Mediated Transcytosis Using Monoclonal Antibody Conjugates.Biomedicines. 2022 Jul 5;10(7):1597. doi: 10.3390/biomedicines10071597. Biomedicines. 2022. PMID: 35884906 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

