Upper and Lower Motor Neuron Degenerations Are Somatotopically Related and Temporally Ordered in the Sod1 Mouse Model of Amyotrophic Lateral Sclerosis
- PMID: 33805792
- PMCID: PMC7998935
- DOI: 10.3390/brainsci11030369
Upper and Lower Motor Neuron Degenerations Are Somatotopically Related and Temporally Ordered in the Sod1 Mouse Model of Amyotrophic Lateral Sclerosis
Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating and fatal neurodegenerative disease arising from the combined degeneration of upper motor neurons (UMN) in the motor cortex, and lower motor neurons (LMN) in the brainstem and spinal cord. This dual impairment raises two major questions: (i) are the degenerations of these two neuronal populations somatotopically related? and if yes (ii), where does neurodegeneration start? If studies carried out on ALS patients clearly demonstrated the somatotopic relationship between UMN and LMN degenerations, their temporal relationship remained an unanswered question. In the present study, we took advantage of the well-described Sod1G86R model of ALS to interrogate the somatotopic and temporal relationships between UMN and LMN degenerations in ALS. Using retrograde labelling from the cervical or lumbar spinal cord of Sod1G86R mice and controls to identify UMN, along with electrophysiology and histology to assess LMN degeneration, we applied rigorous sampling, counting, and statistical analyses, and show that UMN and LMN degenerations are somatotopically related and that UMN depletion precedes LMN degeneration. Together, the data indicate that UMN degeneration is a particularly early and thus relevant event in ALS, in accordance with a possible cortical origin of the disease, and emphasize the need to further elucidate the molecular mechanisms behind UMN degeneration, towards new therapeutic avenues.
Keywords: amyotrophic lateral sclerosis; descending propagation; dying-forward; lower motor neurons; motor cortex; somatotopy; upper motor neurons.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures
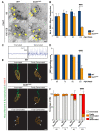
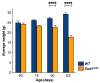
Similar articles
-
The impact of upper and lower motor neuron burden on diagnostic certainty, and clinical course of spinal-onset amyotrophic lateral sclerosis: a cluster-based approach.J Neurol. 2023 Oct;270(10):4868-4875. doi: 10.1007/s00415-023-11827-7. Epub 2023 Jun 20. J Neurol. 2023. PMID: 37338613
-
Amyotrophic lateral sclerosis phenotypes significantly differ in terms of magnetic susceptibility properties of the precentral cortex.Eur Radiol. 2021 Jul;31(7):5272-5280. doi: 10.1007/s00330-020-07547-5. Epub 2021 Jan 5. Eur Radiol. 2021. PMID: 33399906
-
Differential motor neuron involvement in progressive muscular atrophy: a comparative study with amyotrophic lateral sclerosis.BMJ Open. 2014 May 14;4(5):e005213. doi: 10.1136/bmjopen-2014-005213. BMJ Open. 2014. PMID: 24833696 Free PMC article.
-
Amyotrophic lateral sclerosis: lower motor neuron disease spreading to upper motor neurons.Muscle Nerve. 1993 Aug;16(8):864-9. doi: 10.1002/mus.880160810. Muscle Nerve. 1993. PMID: 8332139 Review.
-
[Objective markers for upper motor neuron involvement in amyotrophic lateral sclerosis].Brain Nerve. 2007 Oct;59(10):1053-64. Brain Nerve. 2007. PMID: 17969345 Review. Japanese.
Cited by
-
Differential Epigenetic Signature of Corticospinal Motor Neurons in ALS.Brain Sci. 2021 Jun 7;11(6):754. doi: 10.3390/brainsci11060754. Brain Sci. 2021. PMID: 34200232 Free PMC article.
-
Neural circuit and synaptic dysfunctions in ALS-FTD pathology.Front Neural Circuits. 2023 Jul 4;17:1208876. doi: 10.3389/fncir.2023.1208876. eCollection 2023. Front Neural Circuits. 2023. PMID: 37469832 Free PMC article. Review.
-
Advances in Gene Delivery Methods to Label and Modulate Activity of Upper Motor Neurons: Implications for Amyotrophic Lateral Sclerosis.Brain Sci. 2021 Aug 24;11(9):1112. doi: 10.3390/brainsci11091112. Brain Sci. 2021. PMID: 34573134 Free PMC article. Review.
-
A novel assessment of fine-motor function reveals early hindlimb and detectable forelimb deficits in an experimental model of ALS.Sci Rep. 2022 Oct 11;12(1):17010. doi: 10.1038/s41598-022-20333-1. Sci Rep. 2022. PMID: 36220871 Free PMC article.
-
Neurophysiological Mechanisms Underlying Cortical Hyper-Excitability in Amyotrophic Lateral Sclerosis: A Review.Brain Sci. 2021 Apr 27;11(5):549. doi: 10.3390/brainsci11050549. Brain Sci. 2021. PMID: 33925493 Free PMC article. Review.
References
-
- Bede P., Bodke A., Elamin M., Byrne S., McLaughlin R.L., Jordan N., Hampel H., Gallagher L., Lynch C., Fagan A.J., et al. Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): A neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J. Neurol. Neurosurg. Psychiatry. 2013;84:7166–7773. doi: 10.1136/jnnp-2012-302674. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

