Regulation of Nuclear Mechanics and the Impact on DNA Damage
- PMID: 33804722
- PMCID: PMC8003950
- DOI: 10.3390/ijms22063178
Regulation of Nuclear Mechanics and the Impact on DNA Damage
Abstract
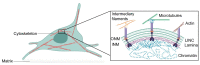
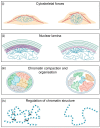
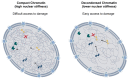
In eukaryotic cells, the nucleus houses the genomic material of the cell. The physical properties of the nucleus and its ability to sense external mechanical cues are tightly linked to the regulation of cellular events, such as gene expression. Nuclear mechanics and morphology are altered in many diseases such as cancer and premature ageing syndromes. Therefore, it is important to understand how different components contribute to nuclear processes, organisation and mechanics, and how they are misregulated in disease. Although, over the years, studies have focused on the nuclear lamina-a mesh of intermediate filament proteins residing between the chromatin and the nuclear membrane-there is growing evidence that chromatin structure and factors that regulate chromatin organisation are essential contributors to the physical properties of the nucleus. Here, we review the main structural components that contribute to the mechanical properties of the nucleus, with particular emphasis on chromatin structure. We also provide an example of how nuclear stiffness can both impact and be affected by cellular processes such as DNA damage and repair.
Keywords: DNA; DNA damage; chromatin; cytoskeleton; lamin; mechanics; nucleus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Separate roles for chromatin and lamins in nuclear mechanics.Nucleus. 2018 Jan 1;9(1):119-124. doi: 10.1080/19491034.2017.1414118. Epub 2017 Dec 28. Nucleus. 2018. PMID: 29227210 Free PMC article.
-
Cytoskeletal prestress regulates nuclear shape and stiffness in cardiac myocytes.Exp Biol Med (Maywood). 2015 Nov;240(11):1543-54. doi: 10.1177/1535370215583799. Epub 2015 Apr 23. Exp Biol Med (Maywood). 2015. PMID: 25908635 Free PMC article.
-
Nuclear lamina at the crossroads of the cytoplasm and nucleus.J Struct Biol. 2012 Jan;177(1):24-31. doi: 10.1016/j.jsb.2011.11.007. Epub 2011 Nov 22. J Struct Biol. 2012. PMID: 22126840 Free PMC article. Review.
-
Towards an integrated understanding of the structure and mechanics of the cell nucleus.Bioessays. 2008 Mar;30(3):226-36. doi: 10.1002/bies.20720. Bioessays. 2008. PMID: 18293361 Review.
-
Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation.Annu Rev Biochem. 2015;84:131-64. doi: 10.1146/annurev-biochem-060614-034115. Epub 2015 Feb 26. Annu Rev Biochem. 2015. PMID: 25747401 Review.
Cited by
-
Natural Deep Eutectic Extracts of Propolis, Sideritis scardica, and Plantago major Reveal Potential Antiageing Activity during Yeast Chronological Lifespan.Oxid Med Cell Longev. 2022 Aug 30;2022:8368717. doi: 10.1155/2022/8368717. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36082083 Free PMC article.
-
The Nucleoskeleton: Crossroad of Mechanotransduction in Skeletal Muscle.Front Physiol. 2021 Oct 15;12:724010. doi: 10.3389/fphys.2021.724010. eCollection 2021. Front Physiol. 2021. PMID: 34721058 Free PMC article. Review.
-
DNA Damage and Nuclear Morphological Changes in Cardiac Hypertrophy Are Mediated by SNRK Through Actin Depolymerization.Circulation. 2023 Nov 14;148(20):1582-1592. doi: 10.1161/CIRCULATIONAHA.123.066002. Epub 2023 Sep 18. Circulation. 2023. PMID: 37721051 Free PMC article.
-
Unique Astrocyte Cytoskeletal and Nuclear Morphology in a Three-Dimensional Tissue-Engineered Rostral Migratory Stream.Neuroglia. 2022 Mar;3(1):41-60. doi: 10.3390/neuroglia3010003. Epub 2022 Mar 6. Neuroglia. 2022. PMID: 36776937 Free PMC article.
-
Nuclear softening mediated by Sun2 suppression delays mechanical stress-induced cellular senescence.Cell Death Discov. 2023 May 17;9(1):167. doi: 10.1038/s41420-023-01467-1. Cell Death Discov. 2023. PMID: 37198162 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

