Quinuclidine-Based Carbamates as Potential CNS Active Compounds
- PMID: 33804719
- PMCID: PMC8003920
- DOI: 10.3390/pharmaceutics13030420
Quinuclidine-Based Carbamates as Potential CNS Active Compounds
Abstract
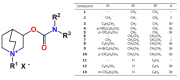
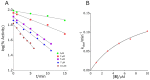
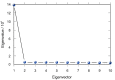
The treatment of central nervous system (CNS) diseases related to the decrease of neurotransmitter acetylcholine in neurons is based on compounds that prevent or disrupt the action of acetylcholinesterase and butyrylcholinesterase. A series of thirteen quinuclidine carbamates were designed using quinuclidine as the structural base and a carbamate group to ensure the covalent binding to the cholinesterase, which were synthesized and tested as potential human acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors. The synthesized compounds differed in the substituents on the amino and carbamoyl parts of the molecule. All of the prepared carbamates displayed a time-dependent inhibition with overall inhibition rate constants in the 103 M-1 min-1 range. None of the compounds showed pronounced selectivity for any of the cholinesterases. The in silico determined ability of compounds to cross the blood-brain barrier (BBB) revealed that six compounds should be able to pass the BBB by passive transport. In addition, the compounds did not show toxicity toward cells that represented the main models of individual organs. By machine learning, the most optimal regression models for the prediction of bioactivity were established and validated. Models for AChE and BChE described 89 and 90% of the total variations among the data, respectively. These models facilitated the prediction and design of new and more potent inhibitors. Altogether, our study confirmed that quinuclidinium carbamates are promising candidates for further development as CNS-active drugs, particularly for Alzheimer's disease treatment.
Keywords: Alzheimer’s disease; acetylcholinesterase; butyrylcholinesterase; covalent binding; cytotoxicity; inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
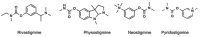



Similar articles
-
Design, Synthesis and Biological Evaluation of Biscarbamates as Potential Selective Butyrylcholinesterase Inhibitors for the Treatment of Alzheimer's Disease.Pharmaceuticals (Basel). 2022 Sep 30;15(10):1220. doi: 10.3390/ph15101220. Pharmaceuticals (Basel). 2022. PMID: 36297332 Free PMC article.
-
N-alkylpiperidine carbamates as potential anti-Alzheimer's agents.Eur J Med Chem. 2020 Jul 1;197:112282. doi: 10.1016/j.ejmech.2020.112282. Epub 2020 Apr 15. Eur J Med Chem. 2020. PMID: 32380361
-
Amino acid residues involved in the interaction of acetylcholinesterase and butyrylcholinesterase with the carbamates Ro 02-0683 and bambuterol, and with terbutaline.Biochim Biophys Acta. 1999 Aug 17;1433(1-2):261-71. doi: 10.1016/s0167-4838(99)00124-7. Biochim Biophys Acta. 1999. PMID: 10446376
-
Proline-Based Carbamates as Cholinesterase Inhibitors.Molecules. 2017 Nov 14;22(11):1969. doi: 10.3390/molecules22111969. Molecules. 2017. PMID: 29135926 Free PMC article.
-
Cholinesterase Inhibitory Activity of Some semi-Rigid Spiro Heterocycles: POM Analyses and Crystalline Structure of Pharmacophore Site.Mini Rev Med Chem. 2018;18(8):711-716. doi: 10.2174/1389557517666170713114039. Mini Rev Med Chem. 2018. PMID: 28714400 Review.
Cited by
-
Profiling Novel Quinuclidine-Based Derivatives as Potential Anticholinesterase Drugs: Enzyme Inhibition and Effects on Cell Viability.Int J Mol Sci. 2023 Dec 21;25(1):155. doi: 10.3390/ijms25010155. Int J Mol Sci. 2023. PMID: 38203326 Free PMC article.
-
Synthesis, Biological Evaluation and Machine Learning Prediction Model for Fluorinated Cinchona Alkaloid-Based Derivatives as Cholinesterase Inhibitors.Pharmaceuticals (Basel). 2022 Sep 30;15(10):1214. doi: 10.3390/ph15101214. Pharmaceuticals (Basel). 2022. PMID: 36297327 Free PMC article.
-
Antimicrobial Activity of Quasi-Enantiomeric Cinchona Alkaloid Derivatives and Prediction Model Developed by Machine Learning.Antibiotics (Basel). 2021 May 31;10(6):659. doi: 10.3390/antibiotics10060659. Antibiotics (Basel). 2021. PMID: 34073082 Free PMC article.
-
4-Aminoquinoline-Based Adamantanes as Potential Anticholinesterase Agents in Symptomatic Treatment of Alzheimer's Disease.Pharmaceutics. 2022 Jun 20;14(6):1305. doi: 10.3390/pharmaceutics14061305. Pharmaceutics. 2022. PMID: 35745878 Free PMC article.
References
-
- World Health Organization . Neurological Disorders: Public Health Challenges. WHO Library Cataloguing in Publication Data. WHO; Geneva, Switzerland: 2019.
-
- Peterson B. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019;15:321–387.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

