Cross-Talk among Polymorphonuclear Neutrophils, Immune, and Non-Immune Cells via Released Cytokines, Granule Proteins, Microvesicles, and Neutrophil Extracellular Trap Formation: A Novel Concept of Biology and Pathobiology for Neutrophils
- PMID: 33803773
- PMCID: PMC8003289
- DOI: 10.3390/ijms22063119
Cross-Talk among Polymorphonuclear Neutrophils, Immune, and Non-Immune Cells via Released Cytokines, Granule Proteins, Microvesicles, and Neutrophil Extracellular Trap Formation: A Novel Concept of Biology and Pathobiology for Neutrophils
Abstract
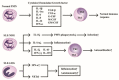
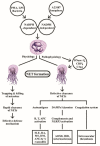
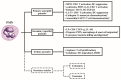
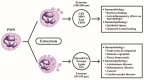
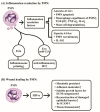
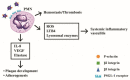
Polymorphonuclear neutrophils (PMNs) are traditionally regarded as professional phagocytic and acute inflammatory cells that engulf the microbial pathogens. However, accumulating data have suggested that PMNs are multi-potential cells exhibiting many important biological functions in addition to phagocytosis. These newly found novel activities of PMN include production of different kinds of cytokines/chemokines/growth factors, release of neutrophil extracellular traps (NET)/ectosomes/exosomes and trogocytosis (membrane exchange) with neighboring cells for modulating innate, and adaptive immune responses. Besides, PMNs exhibit potential heterogeneity and plasticity in involving antibody-dependent cellular cytotoxicity (ADCC), cancer immunity, autoimmunity, inflammatory rheumatic diseases, and cardiovascular diseases. Interestingly, PMNs may also play a role in ameliorating inflammatory reaction and wound healing by a subset of PMN myeloid-derived suppressor cells (PMN-MDSC). Furthermore, PMNs can interact with other non-immune cells including platelets, epithelial and endothelial cells to link hemostasis, mucosal inflammation, and atherogenesis. The release of low-density granulocytes (LDG) from bone marrow initiates systemic autoimmune reaction in systemic lupus erythematosus (SLE). In clinical application, identification of certain PMN phenotypes may become prognostic factors for severe traumatic patients. In the present review, we will discuss these newly discovered biological and pathobiological functions of the PMNs.
Keywords: antibody-dependent cellular cytotoxicity (ADCC); ectosome; exosome; low-density granulocyte; neutrophil extracellular trap (NET); polymorphonuclear myeloid-derived suppressor cell (PMN-MDSC); polymorphonuclear neutrophil; systemic lupus erythematosus (SLE); trogocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
What's wrong with neutrophils in lupus?Clin Exp Rheumatol. 2019 Jul-Aug;37(4):684-693. Epub 2018 Nov 19. Clin Exp Rheumatol. 2019. PMID: 30557133 Review.
-
Molecular Basis for Paradoxical Activities of Polymorphonuclear Neutrophils in Inflammation/Anti-Inflammation, Bactericide/Autoimmunity, Pro-Cancer/Anticancer, and Antiviral Infection/SARS-CoV-II-Induced Immunothrombotic Dysregulation.Biomedicines. 2022 Mar 25;10(4):773. doi: 10.3390/biomedicines10040773. Biomedicines. 2022. PMID: 35453523 Free PMC article. Review.
-
The Neutrophil: Constant Defender and First Responder.Front Immunol. 2020 Sep 24;11:571085. doi: 10.3389/fimmu.2020.571085. eCollection 2020. Front Immunol. 2020. PMID: 33072112 Free PMC article. Review.
-
Downregulation of Microparticle Release and Pro-Inflammatory Properties of Activated Human Polymorphonuclear Neutrophils by LMW Fucoidan.J Innate Immun. 2019;11(4):330-346. doi: 10.1159/000494220. Epub 2018 Dec 17. J Innate Immun. 2019. PMID: 30557873 Free PMC article.
-
A Flow Cytometry-Based Assay for High-Throughput Detection and Quantification of Neutrophil Extracellular Traps in Mixed Cell Populations.Cytometry A. 2019 Mar;95(3):268-278. doi: 10.1002/cyto.a.23672. Epub 2018 Dec 14. Cytometry A. 2019. PMID: 30549398 Free PMC article.
Cited by
-
Could a reduced hemoglobin, albumin, lymphocyte, and platelet (HALP) score predict autoimmune hepatitis and degree of liver fibrosis?Rev Assoc Med Bras (1992). 2024 Jan 26;70(1):e20230905. doi: 10.1590/1806-9282.20230905. eCollection 2024. Rev Assoc Med Bras (1992). 2024. PMID: 38294124 Free PMC article.
-
Integrating osteoimmunology and nanoparticle-based drug delivery systems for enhanced fracture healing.Nanomedicine. 2024 Feb;56:102727. doi: 10.1016/j.nano.2023.102727. Epub 2023 Dec 8. Nanomedicine. 2024. PMID: 38056586 Review.
-
Method Matters: Effect of Purification Technology on Neutrophil Phenotype and Function.Front Immunol. 2022 Feb 10;13:820058. doi: 10.3389/fimmu.2022.820058. eCollection 2022. Front Immunol. 2022. PMID: 35222394 Free PMC article.
-
The Role of KH-Type Splicing Regulatory Protein (KSRP) for Immune Functions and Tumorigenesis.Cells. 2022 Apr 28;11(9):1482. doi: 10.3390/cells11091482. Cells. 2022. PMID: 35563788 Free PMC article. Review.
-
The role and metabolic adaptations of neutrophils in premetastatic niches.Biomark Res. 2023 May 9;11(1):50. doi: 10.1186/s40364-023-00493-6. Biomark Res. 2023. PMID: 37158964 Free PMC article. Review.
References
-
- Kudo C., Yamashita T., Araki A., Terashita M., Watanabe T., Atsumi M., Tamura M., Sendo F. Modulation of In Vivo immune response by selective depletion of neutrophils using a monoclonal antibody, RP-3. I. Inhibition by RP-3 treatment of the priming and effector phases of delayed type hypersensitivity to sheep red blood cells in rats. J. Immunol. 1993;150:3728–3738. - PubMed
-
- Tamura M., Sekiya S., Terashita M., Sendo F. Modulation of the in vivo immune response by selective depletion of neutrophils using a monoclonal antibody, RP-3. III. Enhancement by RP-3 treatment of the anti-sheep red blood cell plaque-forming cell response in rats. J. Immunol. 1994;153:1301–1308. - PubMed
-
- Christoffersson G., Vågesjö E., Vandooren J., Lidén M., Massena S., Reinert R.B., Brissova M., Powers A.C., Opdenakker G., Phillipson M. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120:4653–4662. doi: 10.1182/blood-2012-04-421040. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

