Decrease of IL-5 Production by Naive T Cells Cocultured with IL-18-Producing BCG-Pulsed Dendritic Cells from Patients Allergic to House Dust Mite
- PMID: 33803752
- PMCID: PMC8003153
- DOI: 10.3390/vaccines9030277
Decrease of IL-5 Production by Naive T Cells Cocultured with IL-18-Producing BCG-Pulsed Dendritic Cells from Patients Allergic to House Dust Mite
Abstract
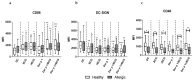
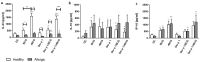
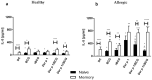
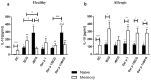
The only currently available anti-tuberculosis vaccine, Bacillus Calmette-Guérin (BCG), has been reported to also protect against unrelated diseases, including inflammatory diseases such as allergic asthma. Recombinant BCG strains that produce IL-18 have been shown to enhance Th1 responses over non-recombinant BCG and to reduce IL-5 production and bronchoalveolar eosinophilia in mice. However, their ability to decrease the immune polarization of human Th2 cells is not known. Here, we show that BCG and recombinant BCG producing human IL-18 (rBCG-hIL-18) induced the maturation of Der p 1-stimulated monocyte-derived dendritic cells (MD-DCs) from healthy controls and from patients allergic to house dust mites. After incubation with mycobacteria and Der p 1, MD-DCs produced significantly more IL-23 and IP-10 but had no effect on IL-12p70 or IL-10 production compared to Der p 1-pulsed MD-DCs in the absence of mycobacteria. In the presence of Der p 1, BCG- and rBCG-hIL-18-pulsed MD-DCs cocultured with naive, but not with memory T cells from allergic patients, resulted in a decrease in IL-5 production compared to non-pulsed MD-DCs cultured in the presence of Der p 1. BCG, and especially rBCG-hIL-18, may thus be potential therapeutic tools to reduce exacerbated Th2 responses in patients with allergic asthma.
Keywords: BCG; Der p 1; Th1/Th2 cells; asthma; dendritic cells; rBCG-hIL-18.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Der p 2 recombinant bacille Calmette-Guérin targets dendritic cells to inhibit allergic airway inflammation in a mouse model of asthma.Respiration. 2013;85(1):49-58. doi: 10.1159/000340007. Epub 2012 Sep 26. Respiration. 2013. PMID: 23018133
-
Immuno-modulatory activity of Ganoderma lucidum-derived polysacharide on human monocytoid dendritic cells pulsed with Der p 1 allergen.BMC Immunol. 2011 May 25;12:31. doi: 10.1186/1471-2172-12-31. BMC Immunol. 2011. PMID: 21612588 Free PMC article.
-
Direct regulatory immune activity of lactic acid bacteria on Der p 1-pulsed dendritic cells from allergic patients.J Allergy Clin Immunol. 2005 Jul;116(1):198-204. doi: 10.1016/j.jaci.2005.02.037. J Allergy Clin Immunol. 2005. PMID: 15990795
-
Th2 polarization by Der p 1--pulsed monocyte-derived dendritic cells is due to the allergic status of the donors.Blood. 2001 Aug 15;98(4):1135-41. doi: 10.1182/blood.v98.4.1135. Blood. 2001. PMID: 11493462
-
Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses.Front Immunol. 2018 Mar 19;9:455. doi: 10.3389/fimmu.2018.00455. eCollection 2018. Front Immunol. 2018. PMID: 29616018 Free PMC article. Review.
Cited by
-
Adjuvants and Antigen-Delivery Systems for Subunit Vaccines against Tuberculosis.Vaccines (Basel). 2021 Aug 30;9(9):972. doi: 10.3390/vaccines9090972. Vaccines (Basel). 2021. PMID: 34579209 Free PMC article.
-
Recombinant BCG to Enhance Its Immunomodulatory Activities.Vaccines (Basel). 2022 May 23;10(5):827. doi: 10.3390/vaccines10050827. Vaccines (Basel). 2022. PMID: 35632582 Free PMC article. Review.
-
CpG-ODN Signaling via Dendritic Cells-Expressing MyD88, but Not IL-10, Inhibits Allergic Sensitization.Vaccines (Basel). 2021 Jul 5;9(7):743. doi: 10.3390/vaccines9070743. Vaccines (Basel). 2021. PMID: 34358159 Free PMC article.
References
-
- Till S., Dickason R., Huston D., Humbert M., Robinson D., Larché M., Durham S., Kay A., Corrigan C. IL-5 secretion by allergen-stimulated CD4+ T cells in primary culture: Relationship to expression of allergic disease. J. Allergy Clin. Immunol. 1997;99:563–569. doi: 10.1016/S0091-6749(97)70085-X. - DOI - PubMed
-
- Ebner C., Schenk S., Najafian N., Siemann U., Steiner R., Fischer G.W., Hoffmann K., Szépfalusi Z., Scheiner O., Kraft D. Nonallergic individuals recognize the same T cell epitopes of Bet v 1, the major birch pollen allergen, as atopic patients. J. Immunol. 1995;154:1932–1940. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

