In Translation: FcRn across the Therapeutic Spectrum
- PMID: 33802650
- PMCID: PMC8002405
- DOI: 10.3390/ijms22063048
In Translation: FcRn across the Therapeutic Spectrum
Abstract
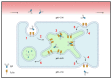
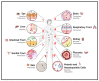
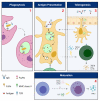
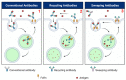
As an essential modulator of IgG disposition, the neonatal Fc receptor (FcRn) governs the pharmacokinetics and functions many therapeutic modalities. In this review, we thoroughly reexamine the hitherto elucidated biological and thermodynamic properties of FcRn to provide context for our assessment of more recent advances, which covers antigen-binding fragment (Fab) determinants of FcRn affinity, transgenic preclinical models, and FcRn targeting as an immune-complex (IC)-clearing strategy. We further comment on therapeutic antibodies authorized for treating SARS-CoV-2 (bamlanivimab, casirivimab, and imdevimab) and evaluate their potential to saturate FcRn-mediated recycling. Finally, we discuss modeling and simulation studies that probe the quantitative relationship between in vivo IgG persistence and in vitro FcRn binding, emphasizing the importance of endosomal transit parameters.
Keywords: Fc-fusion protein; immune complex; immunoglobulin G; monoclonal antibody; neonatal Fc receptor; pharmacokinetics; physiologically-based pharmacokinetic modeling.
Conflict of interest statement
T.Q. owns equity in Humanigen, a clinical-stage biopharmaceutical company.
Figures
Similar articles
-
Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity?Mol Immunol. 2013 Dec;56(4):660-74. doi: 10.1016/j.molimm.2013.05.008. Epub 2013 Aug 2. Mol Immunol. 2013. PMID: 23917469 Review.
-
Changes in complementarity-determining regions significantly alter IgG binding to the neonatal Fc receptor (FcRn) and pharmacokinetics.MAbs. 2018 Jan;10(1):81-94. doi: 10.1080/19420862.2017.1389355. Epub 2017 Nov 3. MAbs. 2018. PMID: 28991504 Free PMC article.
-
A minimal physiologically based pharmacokinetic model to investigate FcRn-mediated monoclonal antibody salvage: Effects of Kon, Koff, endosome trafficking, and animal species.MAbs. 2018 Nov-Dec;10(8):1322-1331. doi: 10.1080/19420862.2018.1506648. Epub 2018 Sep 11. MAbs. 2018. PMID: 30130450 Free PMC article.
-
The Neonatal Fc Receptor (FcRn): A Misnomer?Front Immunol. 2019 Jul 10;10:1540. doi: 10.3389/fimmu.2019.01540. eCollection 2019. Front Immunol. 2019. PMID: 31354709 Free PMC article. Review.
-
Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences.Drug Metab Dispos. 2011 Sep;39(9):1469-77. doi: 10.1124/dmd.111.039453. Epub 2011 May 24. Drug Metab Dispos. 2011. PMID: 21610128
Cited by
-
The therapeutic age of the neonatal Fc receptor.Nat Rev Immunol. 2023 Jul;23(7):415-432. doi: 10.1038/s41577-022-00821-1. Epub 2023 Feb 1. Nat Rev Immunol. 2023. PMID: 36726033 Free PMC article. Review.
-
The Neonatal Fc Receptor Is Elevated in Monocyte-Derived Immune Cells in Pancreatic Cancer.Int J Mol Sci. 2022 Jun 25;23(13):7066. doi: 10.3390/ijms23137066. Int J Mol Sci. 2022. PMID: 35806069 Free PMC article.
-
Extended Half-life Antibodies: A Narrative Review of a New Approach in the Management of Atopic Dermatitis.Dermatol Ther (Heidelb). 2024 Sep;14(9):2393-2406. doi: 10.1007/s13555-024-01253-6. Epub 2024 Aug 15. Dermatol Ther (Heidelb). 2024. PMID: 39147994 Free PMC article. Review.
-
Insight into the avidity-affinity relationship of the bivalent, pH-dependent interaction between IgG and FcRn.MAbs. 2024 Jan-Dec;16(1):2361585. doi: 10.1080/19420862.2024.2361585. Epub 2024 Jun 7. MAbs. 2024. PMID: 38849969 Free PMC article.
-
Monoclonal Antibody Engineering and Design to Modulate FcRn Activities: A Comprehensive Review.Int J Mol Sci. 2022 Aug 24;23(17):9604. doi: 10.3390/ijms23179604. Int J Mol Sci. 2022. PMID: 36077002 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

