Chronicles of Nanoerythrosomes: An Erythrocyte-Based Biomimetic Smart Drug Delivery System as a Therapeutic and Diagnostic Tool in Cancer Therapy
- PMID: 33802156
- PMCID: PMC7998655
- DOI: 10.3390/pharmaceutics13030368
Chronicles of Nanoerythrosomes: An Erythrocyte-Based Biomimetic Smart Drug Delivery System as a Therapeutic and Diagnostic Tool in Cancer Therapy
Abstract
Recently, drug delivery using natural biological carriers has emerged as one of the most widely investigated topics of research. Erythrocytes, or red blood cells, can act as potential carriers for a wide variety of drugs, including anticancer, antibacterial, antiviral, and anti-inflammatory, along with various proteins, peptides, enzymes, and other macromolecules. The red blood cell-based nanocarrier systems, also called nanoerythrosomes, are nanovesicles poised with extraordinary features such as long blood circulation times, the ability to escape immune system, the ability to release the drug gradually, the protection of drugs from various endogenous factors, targeted and specified delivery of drugs, as well as possessing both therapeutic and diagnostic applications in various fields of biomedical sciences. Their journey over the last two decades is escalating with fast pace, ranging from in vivo to preclinical and clinical studies by encapsulating a number of drugs into these carriers. Being biomimetic nanoparticles, they have enhanced the stability profile of drugs and their excellent site-specific targeting ability makes them potential carrier systems in the diagnosis and therapy of wide variety of tumors including gliomas, lung cancers, breast cancers, colon cancers, gastric cancers, and other solid tumors. This review focuses on the most recent advancements in the field of nanoerythrosomes, as an excellent and promising nanoplatform for the novel drug delivery of various drugs particularly antineoplastic drugs along with their potential as a promising diagnostic tool for the identification of different tumors.
Keywords: biomimetic; cancer therapy; diagnostics; imaging agents; nanoerythrocyte; nanoerythrosome; nanovesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

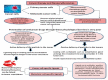
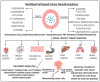
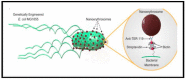
Similar articles
-
Erythrocytes as Carriers: From Drug Delivery to Biosensors.Pharmaceutics. 2020 Mar 18;12(3):276. doi: 10.3390/pharmaceutics12030276. Pharmaceutics. 2020. PMID: 32197542 Free PMC article. Review.
-
Light-Triggered Biomimetic Nanoerythrocyte for Tumor-Targeted Lung Metastatic Combination Therapy of Malignant Melanoma.Small. 2018 Sep;14(38):e1801754. doi: 10.1002/smll.201801754. Epub 2018 Aug 23. Small. 2018. PMID: 30141569
-
Metformin-loaded nanoerythrosomes: An erythrocyte-based drug delivery system as a therapeutic tool for glioma.Heliyon. 2023 Jun 7;9(6):e17082. doi: 10.1016/j.heliyon.2023.e17082. eCollection 2023 Jun. Heliyon. 2023. PMID: 37484272 Free PMC article.
-
Nanoerythrosome, a new derivative of erythrocyte ghost: preparation and antineoplastic potential as drug carrier for daunorubicin.Anticancer Res. 1994 May-Jun;14(3A):915-9. Anticancer Res. 1994. PMID: 8074493
-
Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
Cited by
-
Cell membrane-coated nanoparticles: a novel multifunctional biomimetic drug delivery system.Drug Deliv Transl Res. 2023 Mar;13(3):716-737. doi: 10.1007/s13346-022-01252-0. Epub 2022 Nov 22. Drug Deliv Transl Res. 2023. PMID: 36417162 Free PMC article. Review.
-
Novel Anticancer Strategies II.Pharmaceutics. 2023 Feb 10;15(2):605. doi: 10.3390/pharmaceutics15020605. Pharmaceutics. 2023. PMID: 36839927 Free PMC article.
-
Bioinspired Platelet-like Nanovector for Enhancing Cancer Therapy via P-Selectin Targeting.Pharmaceutics. 2022 Nov 26;14(12):2614. doi: 10.3390/pharmaceutics14122614. Pharmaceutics. 2022. PMID: 36559108 Free PMC article.
-
The role of erythrocytes and erythroid progenitor cells in tumors.Open Life Sci. 2022 Dec 15;17(1):1641-1656. doi: 10.1515/biol-2022-0102. eCollection 2022. Open Life Sci. 2022. PMID: 36567722 Free PMC article. Review.
-
Recent advances in the liposomal nanovesicles based immunotherapy in the treatment of cancer: A review.Saudi Pharm J. 2023 Feb;31(2):279-294. doi: 10.1016/j.jsps.2022.12.008. Epub 2022 Dec 24. Saudi Pharm J. 2023. PMID: 36942270 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

