Lipid Metabolism and Ferroptosis
- PMID: 33801564
- PMCID: PMC8000263
- DOI: 10.3390/biology10030184
Lipid Metabolism and Ferroptosis
Abstract
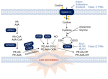
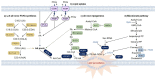
Ferroptosis is a type of iron-dependent regulated necrosis induced by lipid peroxidation that occurs in cellular membranes. Among the various lipids, polyunsaturated fatty acids (PUFAs) associated with several phospholipids, such as phosphatidylethanolamine (PE) and phosphatidylcholine (PC), are responsible for ferroptosis-inducing lipid peroxidation. Since the de novo synthesis of PUFAs is strongly restricted in mammals, cells take up essential fatty acids from the blood and lymph to produce a variety of PUFAs via PUFA biosynthesis pathways. Free PUFAs can be incorporated into the cellular membrane by several enzymes, such as ACLS4 and LPCAT3, and undergo lipid peroxidation through enzymatic and non-enzymatic mechanisms. These pathways are tightly regulated by various metabolic and signaling pathways. In this review, we summarize our current knowledge of how various lipid metabolic pathways are associated with lipid peroxidation and ferroptosis. Our review will provide insight into treatment strategies for ferroptosis-related diseases.
Keywords: GPX4; ferroptosis; lipid peroxidation; lipoxygenase; polyunsaturated fatty acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A tale of two lipids: Lipid unsaturation commands ferroptosis sensitivity.Proteomics. 2023 Mar;23(6):e2100308. doi: 10.1002/pmic.202100308. Epub 2023 Jan 13. Proteomics. 2023. PMID: 36398995 Review.
-
Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis.Proc Natl Acad Sci U S A. 2016 Aug 23;113(34):E4966-75. doi: 10.1073/pnas.1603244113. Epub 2016 Aug 9. Proc Natl Acad Sci U S A. 2016. PMID: 27506793 Free PMC article.
-
FAF1 blocks ferroptosis by inhibiting peroxidation of polyunsaturated fatty acids.Proc Natl Acad Sci U S A. 2022 Apr 26;119(17):e2107189119. doi: 10.1073/pnas.2107189119. Epub 2022 Apr 25. Proc Natl Acad Sci U S A. 2022. PMID: 35467977 Free PMC article.
-
Polyunsaturated Fatty Acids Drive Lipid Peroxidation during Ferroptosis.Cells. 2023 Mar 4;12(5):804. doi: 10.3390/cells12050804. Cells. 2023. PMID: 36899940 Free PMC article. Review.
-
Therapy-induced lipid uptake and remodeling underpin ferroptosis hypersensitivity in prostate cancer.Cancer Metab. 2020 Jun 19;8:11. doi: 10.1186/s40170-020-00217-6. eCollection 2020. Cancer Metab. 2020. PMID: 32577235 Free PMC article.
Cited by
-
Exploring the relationship between anastasis and mitochondrial ROS-mediated ferroptosis in metastatic chemoresistant cancers: a call for investigation.Front Immunol. 2024 Jul 2;15:1428920. doi: 10.3389/fimmu.2024.1428920. eCollection 2024. Front Immunol. 2024. PMID: 39015566 Free PMC article. Review.
-
Targeting ferroptosis and ferritinophagy: new targets for cardiovascular diseases.J Zhejiang Univ Sci B. 2024 Jan 15;25(1):1-22. doi: 10.1631/jzus.B2300097. J Zhejiang Univ Sci B. 2024. PMID: 38163663 Free PMC article. Review.
-
Phytochemicals Targeting Ferroptosis: Therapeutic Opportunities and Prospects for Treating Breast Cancer.Pharmaceuticals (Basel). 2022 Nov 5;15(11):1360. doi: 10.3390/ph15111360. Pharmaceuticals (Basel). 2022. PMID: 36355532 Free PMC article. Review.
-
Ferroptosis: A New Development Trend in Periodontitis.Cells. 2022 Oct 24;11(21):3349. doi: 10.3390/cells11213349. Cells. 2022. PMID: 36359745 Free PMC article. Review.
-
Ferroptosis in Hepatocellular Carcinoma: Mechanisms, Drug Targets and Approaches to Clinical Translation.Cancers (Basel). 2022 Apr 4;14(7):1826. doi: 10.3390/cancers14071826. Cancers (Basel). 2022. PMID: 35406596 Free PMC article. Review.
References
-
- Ardanaz N., Yang X.-P., Cifuentes M.E., Haurani M.J., Jackson K.W., Liao T.-D., Carretero O.A., Pagano P.J. Lack of Glutathione Peroxidase 1 Accelerates Cardiac-Specific Hypertrophy and Dysfunction in Angiotensin II Hypertension. Hypertension. 2010;55:116–123. doi: 10.1161/HYPERTENSIONAHA.109.135715. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

