Interactions of SARS-CoV-2 with the Blood-Brain Barrier
- PMID: 33800954
- PMCID: PMC7961671
- DOI: 10.3390/ijms22052681
Interactions of SARS-CoV-2 with the Blood-Brain Barrier
Abstract
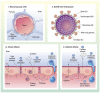
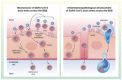
Emerging data indicate that neurological complications occur as a consequence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The blood-brain barrier (BBB) is a critical interface that regulates entry of circulating molecules into the CNS, and is regulated by signals that arise from the brain and blood compartments. In this review, we discuss mechanisms by which SARS-CoV-2 interactions with the BBB may contribute to neurological dysfunction associated with coronavirus disease of 2019 (COVID-19), which is caused by SARS-CoV-2. We consider aspects of peripheral disease, such as hypoxia and systemic inflammatory response syndrome/cytokine storm, as well as CNS infection and mechanisms of viral entry into the brain. We also discuss the contribution of risk factors for developing severe COVID-19 to BBB dysfunction that could increase viral entry or otherwise damage the brain.
Keywords: APOE; COVID-19; SARS-CoV-2; blood–brain barrier; brain; hypoxia; inflammation; thrombosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
SARS-CoV-2 journey to the brain with a focus on potential role of docosahexaenoic acid bioactive lipid mediators.Biochimie. 2021 May;184:95-103. doi: 10.1016/j.biochi.2021.02.012. Epub 2021 Feb 25. Biochimie. 2021. PMID: 33639198 Free PMC article. Review.
-
Neurological Complications Associated with the Blood-Brain Barrier Damage Induced by the Inflammatory Response During SARS-CoV-2 Infection.Mol Neurobiol. 2021 Feb;58(2):520-535. doi: 10.1007/s12035-020-02134-7. Epub 2020 Sep 25. Mol Neurobiol. 2021. PMID: 32978729 Free PMC article. Review.
-
Possible routes of SARS-CoV-2 invasion in brain: In context of neurological symptoms in COVID-19 patients.J Neurosci Res. 2020 Dec;98(12):2376-2383. doi: 10.1002/jnr.24717. Epub 2020 Aug 31. J Neurosci Res. 2020. PMID: 32869376 Review.
-
Neurological manifestations of SARS-CoV-2: complexity, mechanism and associated disorders.Eur J Med Res. 2023 Aug 30;28(1):307. doi: 10.1186/s40001-023-01293-2. Eur J Med Res. 2023. PMID: 37649125 Free PMC article. Review.
-
COVID-19-Associated Neurological Disorders: The Potential Route of CNS Invasion and Blood-Brain Relevance.Cells. 2020 Oct 27;9(11):2360. doi: 10.3390/cells9112360. Cells. 2020. PMID: 33120941 Free PMC article. Review.
Cited by
-
COVID-19 and cognitive impairment: neuroinvasive and blood‒brain barrier dysfunction.J Neuroinflammation. 2022 Sep 7;19(1):222. doi: 10.1186/s12974-022-02579-8. J Neuroinflammation. 2022. PMID: 36071466 Free PMC article. Review.
-
Can SARS-CoV-2 Infection Lead to Neurodegeneration and Parkinson's Disease?Brain Sci. 2021 Dec 18;11(12):1654. doi: 10.3390/brainsci11121654. Brain Sci. 2021. PMID: 34942956 Free PMC article. Review.
-
Human Brain In Vitro Model for Pathogen Infection-Related Neurodegeneration Study.Int J Mol Sci. 2024 Jun 13;25(12):6522. doi: 10.3390/ijms25126522. Int J Mol Sci. 2024. PMID: 38928228 Free PMC article. Review.
-
COVID-19 and neurodegeneration: The mitochondrial connection.Aging Cell. 2022 Nov;21(11):e13727. doi: 10.1111/acel.13727. Epub 2022 Oct 11. Aging Cell. 2022. PMID: 36219531 Free PMC article.
-
Blood-brain Barrier Damage is Pivotal for SARS-CoV-2 Infection to the Central Nervous System.Exp Neurobiol. 2022 Aug 31;31(4):270-276. doi: 10.5607/en21049. Exp Neurobiol. 2022. PMID: 36050226 Free PMC article.
References
-
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. - DOI - PMC - PubMed
-
- Varatharaj A., Thomas N., Ellul M.A., Davies N.W.S., Pollak T.A., Tenorio E.L., Sultan M., Easton A., Breen G., Zandi M., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

