Engineering of Challenging G Protein-Coupled Receptors for Structure Determination and Biophysical Studies
- PMID: 33800379
- PMCID: PMC7962830
- DOI: 10.3390/molecules26051465
Engineering of Challenging G Protein-Coupled Receptors for Structure Determination and Biophysical Studies
Abstract
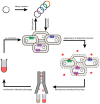
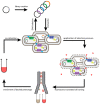
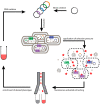
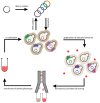
Membrane proteins such as G protein-coupled receptors (GPCRs) exert fundamental biological functions and are involved in a multitude of physiological responses, making these receptors ideal drug targets. Drug discovery programs targeting GPCRs have been greatly facilitated by the emergence of high-resolution structures and the resulting opportunities to identify new chemical entities through structure-based drug design. To enable the determination of high-resolution structures of GPCRs, most receptors have to be engineered to overcome intrinsic hurdles such as their poor stability and low expression levels. In recent years, multiple engineering approaches have been developed to specifically address the technical difficulties of working with GPCRs, which are now beginning to make more challenging receptors accessible to detailed studies. Importantly, successfully engineered GPCRs are not only valuable in X-ray crystallography, but further enable biophysical studies with nuclear magnetic resonance spectroscopy, surface plasmon resonance, native mass spectrometry, and fluorescence anisotropy measurements, all of which are important for the detailed mechanistic understanding, which is the prerequisite for successful drug design. Here, we summarize engineering strategies based on directed evolution to reduce workload and enable biophysical experiments of particularly challenging GPCRs.
Keywords: G protein-coupled receptors; NK1R; NTS1R; PTH1R; directed evolution; protein engineering.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

Similar articles
-
Illuminating the Path to Target GPCR Structures and Functions.Biochemistry. 2020 Oct 13;59(40):3783-3795. doi: 10.1021/acs.biochem.0c00606. Epub 2020 Sep 29. Biochemistry. 2020. PMID: 32956586
-
G Protein-Coupled Receptors - Targets for Fragment-based Drug Discovery.Curr Top Med Chem. 2015;15(24):2523-7. doi: 10.2174/1568026615666150701113151. Curr Top Med Chem. 2015. PMID: 26126904 Review.
-
Recent advances in the determination of G protein-coupled receptor structures.Curr Opin Struct Biol. 2018 Aug;51:28-34. doi: 10.1016/j.sbi.2018.03.002. Epub 2018 Mar 13. Curr Opin Struct Biol. 2018. PMID: 29547818 Review.
-
Successful Strategies to Determine High-Resolution Structures of GPCRs.Trends Pharmacol Sci. 2016 Dec;37(12):1055-1069. doi: 10.1016/j.tips.2016.09.009. Epub 2016 Oct 7. Trends Pharmacol Sci. 2016. PMID: 27726881 Review.
-
Surface plasmon resonance analysis of seven-transmembrane receptors.Methods Enzymol. 2015;556:499-525. doi: 10.1016/bs.mie.2015.01.016. Epub 2015 Mar 21. Methods Enzymol. 2015. PMID: 25857797 Review.
Cited by
-
Thermodynamic architecture and conformational plasticity of GPCRs.Nat Commun. 2023 Jan 9;14(1):128. doi: 10.1038/s41467-023-35790-z. Nat Commun. 2023. PMID: 36624096 Free PMC article.
-
Target-based drug discovery: Applications of fluorescence techniques in high throughput and fragment-based screening.Heliyon. 2023 Dec 19;10(1):e23864. doi: 10.1016/j.heliyon.2023.e23864. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38226204 Free PMC article. Review.
-
Yeast-based directed-evolution for high-throughput structural stabilization of G protein-coupled receptors (GPCRs).Sci Rep. 2022 May 23;12(1):8657. doi: 10.1038/s41598-022-12731-2. Sci Rep. 2022. PMID: 35606532 Free PMC article.
-
Universal platform for the generation of thermostabilized GPCRs that crystallize in LCP.Nat Protoc. 2022 Mar;17(3):698-726. doi: 10.1038/s41596-021-00660-9. Epub 2022 Feb 9. Nat Protoc. 2022. PMID: 35140409 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

