Aptamer Detection of Mycobaterium tuberculosis Mannose-Capped Lipoarabinomannan in Lesion Tissues for Tuberculosis Diagnosis
- PMID: 33791241
- PMCID: PMC8006938
- DOI: 10.3389/fcimb.2021.634915
Aptamer Detection of Mycobaterium tuberculosis Mannose-Capped Lipoarabinomannan in Lesion Tissues for Tuberculosis Diagnosis
Abstract
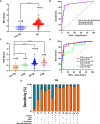
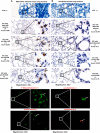
Tuberculosis (TB) is the leading infectious cause of mortality worldwide. However, the diagnosis of TB, especially extrapulmonary TB (EPTB) diagnosis from lesion tissues, remains a challenge. Nucleic acid aptamers are analogous to antibodies and have advantages of easier modification, high specificity, and affinity. Mannose-capped lipoarabinomannan (ManLAM) is a unique surface lipoglycan component or constantly released from mycobacterium tuberculosis (M.tb) cell wall, which makes it a perfect candidate biomarker for TB diagnosis. Our present study aims to establish M.tb ManLAM aptamer-based immunohistochemistry (IHC) method for TB diagnosis. We performed TB diagnosis using 263 formalin-fixed paraffin-embedded tissue samples including 213 TB samples (pulmonary TB (PTB) and EPTB), and 8 samples from latent TB infection (LTBI) high risk subjects, and 42 samples from other non-TB patients with ManLAM aptamer-based IHC and routine laboratory TB diagnostic methods parallelly. The sensitivity and specificity of the ManLAM aptamer-based IHC were 86.38% and 92.86%, with much higher sensitivity than those of mycobacterial culture (9.66%) and acid-fast staining (AFS) (43.01%) and comparability to Interferon-gamma Release Assay (IGRA) (84.38%) and GeneXpert (79.31%). High agreement between ManLAM based-IHC and IGRA or GeneXpert for TB diagnosis were observed. Furthermore, ManLAM aptamer-based IHC combination with other routine TB laboratory diagnostic methods significantly increased the sensitivity up to 88.64%-97.92%. As our knowledge, this is the first report about aptamer-based IHC for disease diagnosis. Thus, ManLAM aptamer-based IHC has potentials for TB diagnosis, including PTB, and EPTB, and assists the diagnosis of LTBI with high effectiveness, feasibility, and easy production.
Keywords: ManLAM; aptamer; diagnosis; lesion tissue; tuberculosis.
Copyright © 2021 Zhou, Xiong, Chen, Wan, Kong, Rao, Xie, Huang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Generation and application of ssDNA aptamers against glycolipid antigen ManLAM of Mycobacterium tuberculosis for TB diagnosis.J Infect. 2016 May;72(5):573-86. doi: 10.1016/j.jinf.2016.01.014. Epub 2016 Feb 3. J Infect. 2016. PMID: 26850356
-
Aptamer against mannose-capped lipoarabinomannan inhibits virulent Mycobacterium tuberculosis infection in mice and rhesus monkeys.Mol Ther. 2014 May;22(5):940-51. doi: 10.1038/mt.2014.31. Epub 2014 Feb 27. Mol Ther. 2014. PMID: 24572295 Free PMC article.
-
Detection of the tuberculosis biomarker mannose-capped lipoarabinomannan in human serum: Impact of sample pretreatment with perchloric acid.Anal Chim Acta. 2019 Jan 10;1046:140-147. doi: 10.1016/j.aca.2018.09.037. Epub 2018 Sep 18. Anal Chim Acta. 2019. PMID: 30482291 Free PMC article.
-
Mycobacterial mannose-capped lipoarabinomannan: a modulator bridging innate and adaptive immunity.Emerg Microbes Infect. 2019;8(1):1168-1177. doi: 10.1080/22221751.2019.1649097. Emerg Microbes Infect. 2019. PMID: 31379262 Free PMC article. Review.
-
Mannose-capped lipoarabinomannan in Mycobacterium tuberculosis pathogenesis.Pathog Dis. 2018 Jun 1;76(4):fty026. doi: 10.1093/femspd/fty026. Pathog Dis. 2018. PMID: 29722821 Free PMC article. Review.
Cited by
-
Aptamers: precision tools for diagnosing and treating infectious diseases.Front Cell Infect Microbiol. 2024 Sep 25;14:1402932. doi: 10.3389/fcimb.2024.1402932. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39386170 Free PMC article. Review.
-
Advancements in Chronic Myeloid Leukemia detection: Development and evaluation of a novel QCM aptasensor for use in clinical practice.Biochem Biophys Rep. 2024 Aug 23;39:101816. doi: 10.1016/j.bbrep.2024.101816. eCollection 2024 Sep. Biochem Biophys Rep. 2024. PMID: 39263318 Free PMC article.
-
New Insights into Aptamers: An Alternative to Antibodies in the Detection of Molecular Biomarkers.Int J Mol Sci. 2024 Jun 21;25(13):6833. doi: 10.3390/ijms25136833. Int J Mol Sci. 2024. PMID: 38999943 Free PMC article. Review.
References
-
- Calligaro G. L., Zijenah L. S., Peter J. G., Theron G., Buser V., McNerney R., et al. . (2017). Effect of new tuberculosis diagnostic technologies on community-based intensified case finding: a multicentre randomised controlled trial. Lancet Infect. Dis. 17, 441–450. 10.1016/S1473-3099(16)30384-X - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

