Interleukin-22 Plays a Protective Role by Regulating the JAK2-STAT3 Pathway to Improve Inflammation, Oxidative Stress, and Neuronal Apoptosis following Cerebral Ischemia-Reperfusion Injury
- PMID: 33790691
- PMCID: PMC7984880
- DOI: 10.1155/2021/6621296
Interleukin-22 Plays a Protective Role by Regulating the JAK2-STAT3 Pathway to Improve Inflammation, Oxidative Stress, and Neuronal Apoptosis following Cerebral Ischemia-Reperfusion Injury
Abstract
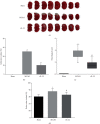
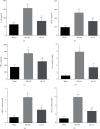
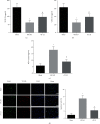
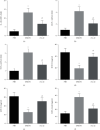
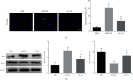
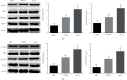
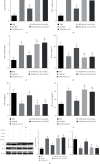
The interleukins (ILs) are a pluripotent cytokine family that have been reported to regulate ischemic stroke and cerebral ischemia/reperfusion (I/R) injury. IL-22 is a member of the IL-10 superfamily and plays important roles in tissue injury and repair. However, the effects of IL-22 on ischemic stroke and cerebral I/R injury remain unclear. In the current study, we provided direct evidence that IL-22 treatment decreased infarct size, neurological deficits, and brain water content in mice subjected to cerebral I/R injury. IL-22 treatment remarkably reduced the expression of inflammatory cytokines, including IL-1β, monocyte chemotactic protein- (MCP-) 1, and tumor necrosis factor- (TNF-) α, both in serum and the ischemic cerebral cortex. In addition, IL-22 treatment also decreased oxidative stress and neuronal apoptosis in mice after cerebral I/R injury. Moreover, IL-22 treatment significantly increased Janus tyrosine kinase (JAK) 2 and signal transducer and activator of transcription (STAT) 3 phosphorylation levels in mice and PC12 cells, and STAT3 knockdown abolished the IL-22-mediated neuroprotective function. These findings suggest that IL-22 might be exploited as a potential therapeutic agent for ischemic stroke and cerebral I/R injury.
Copyright © 2021 Yongfei Dong et al.
Conflict of interest statement
No conflicts of interests are declared by the authors.
Figures
Similar articles
-
Melatonin Plays a Protective Role by Regulating miR-26a-5p-NRSF and JAK2-STAT3 Pathway to Improve Autophagy, Inflammation and Oxidative Stress of Cerebral Ischemia-Reperfusion Injury.Drug Des Devel Ther. 2020 Aug 6;14:3177-3188. doi: 10.2147/DDDT.S262121. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32821085 Free PMC article.
-
microRNA-1906 protects cerebral ischemic injury through activating Janus kinase 2/signal transducer and activator of transcription 3 pathway in rats.Neuroreport. 2020 Aug 12;31(12):871-878. doi: 10.1097/WNR.0000000000001456. Neuroreport. 2020. PMID: 32427806
-
Anti-IL-23 exerted protective effects on cerebral ischemia-reperfusion injury through JAK2/STAT3 signaling pathway.Mol Biol Rep. 2021 Apr;48(4):3475-3484. doi: 10.1007/s11033-021-06339-4. Epub 2021 Apr 26. Mol Biol Rep. 2021. PMID: 33904141
-
The bidirectional role of the JAK2/STAT3 signaling pathway and related mechanisms in cerebral ischemia-reperfusion injury.Exp Neurol. 2021 Jul;341:113690. doi: 10.1016/j.expneurol.2021.113690. Epub 2021 Mar 31. Exp Neurol. 2021. PMID: 33798563 Review.
-
The role of JAK/STAT signaling pathway in cerebral ischemia-reperfusion injury and the therapeutic effect of traditional Chinese medicine: A narrative review.Medicine (Baltimore). 2023 Nov 17;102(46):e35890. doi: 10.1097/MD.0000000000035890. Medicine (Baltimore). 2023. PMID: 37986307 Free PMC article. Review.
Cited by
-
Cav3.2 channel regulates cerebral ischemia/reperfusion injury: a promising target for intervention.Neural Regen Res. 2024 Nov 1;19(11):2480-2487. doi: 10.4103/1673-5374.390966. Epub 2023 Dec 15. Neural Regen Res. 2024. PMID: 38526284 Free PMC article.
-
Reveal the correlation between hub hypoxia/immune-related genes and immunity and diagnosis, and the effect of SAP30 on cell apoptosis, ROS and MDA production in cerebral ischemic stroke.Aging (Albany NY). 2023 Dec 27;15(24):15161-15182. doi: 10.18632/aging.205339. Epub 2023 Dec 27. Aging (Albany NY). 2023. PMID: 38154101 Free PMC article.
-
Effect of trimetazidine against ovarian ischemia/reperfusion injury in rat model: A new pathway: JAK2/STAT3.Iran J Basic Med Sci. 2023;26(11):1370-1379. doi: 10.22038/IJBMS.2023.72544.15776. Iran J Basic Med Sci. 2023. PMID: 37886007 Free PMC article.
-
Phenothiazine Inhibits Neuroinflammation and Inflammasome Activation Independent of Hypothermia After Ischemic Stroke.Mol Neurobiol. 2021 Dec;58(12):6136-6152. doi: 10.1007/s12035-021-02542-3. Epub 2021 Aug 29. Mol Neurobiol. 2021. PMID: 34455546
-
Ciprofol attenuates the isoproterenol-induced oxidative damage, inflammatory response and cardiomyocyte apoptosis.Front Pharmacol. 2022 Nov 22;13:1037151. doi: 10.3389/fphar.2022.1037151. eCollection 2022. Front Pharmacol. 2022. PMID: 36483733 Free PMC article.
References
-
- Xiao G., Lyu M., Wang Y., et al. Ginkgo flavonol glycosides or ginkgolides tend to differentially protect myocardial or cerebral ischemia-reperfusion injury via regulation of TWEAK-Fn14 signaling in heart and brain. Frontiers in Pharmacology. 2019;10:p. 735. doi: 10.3389/fphar.2019.00735. - DOI - PMC - PubMed
-
- Khan M. S., Khan A., Ahmad S., et al. Inhibition of JNK alleviates chronic hypoperfusion-related ischemia induces oxidative stress and brain degeneration via Nrf2/HO-1 and NF-κB signaling. Oxidative Medicine and Cellular Longevity. 2020;2020:18. doi: 10.1155/2020/5291852.5291852 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

