Tocilizumab: An Effective Therapy for Severely and Critically Ill COVID-19 Patients
- PMID: 33790504
- PMCID: PMC7991771
- DOI: 10.5005/jp-journals-10071-23747
Tocilizumab: An Effective Therapy for Severely and Critically Ill COVID-19 Patients
Abstract
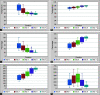
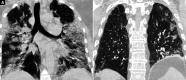
Background: Tocilizumab (TCZ), a monoclonal antibody against the most prevalent cytokine interleukin-6 (IL-6), is an emerging therapeutic option for COVID-19 infections. The present study was undertaken to assess the therapeutic response of TCZ therapy in severely or critically ill COVID-19 patients and its role as an effective modality of management. Methods: The present retrospective observational study included 30 admitted severely or critically ill COVID-19 patients, treated with TCZ therapy on behalf of raised IL-6 levels. The patients' data concerning medical history, clinical manifestation, arterial blood gas analysis, mode of oxygenation, radiological imaging, and outcome were extracted from their medical records and compared pre- and post-TCZ infusion. Results: All patients of the study group had symptomatic presentations with a mean PaO2/FiO2 (P/F) ratio of 205.41 before TCZ infusion. All patients had a raised IL-6 level (mean value 206.56 pg/mL) that was extremely elevated in 90% of patients. Infusion of TCZ dramatically reduced mean body temperature (100.78-99.32°F) and the requirement for supplemental oxygen (68-48%) and improved mean SpO2 (86-89%) and mean P/F ratio (208-240) within 24 hours. Three patients on noninvasive ventilation were weaned off after TCZ infusion. Serum levels of IL-6 were raised initially but declined within 3-5 days of post-TCZ infusion. Conclusion: TCZ appears to be an effective therapeutic option in severely or critically ill COVID-19 patients with raised IL-6 levels. TCZ immediately improves the clinical status of patients by a probable mechanism of inhibition of cytokine storm and reduces COVID-19-related mortalities. How to cite this article: Bhandari S, Rankawat G, Singh A. Tocilizumab: An Effective Therapy for Severely and Critically Ill COVID-19 Patients. Indian J Crit Care Med 2021;25(3):260-266.
Keywords: COVID-19; Cytokine storm; Interleukin-6; Tocilizumab.
Copyright © 2021; Jaypee Brothers Medical Publishers (P) Ltd.
Conflict of interest statement
Source of support: Nil Conflict of interest: None
Figures
Similar articles
-
Tocilizumab treatment in COVID-19: A single center experience.J Med Virol. 2020 Jul;92(7):814-818. doi: 10.1002/jmv.25801. Epub 2020 Apr 15. J Med Virol. 2020. PMID: 32253759 Free PMC article.
-
The role of tocilizumab therapy in critically ill patients with severe acute respiratory syndrome coronavirus 2.J Osteopath Med. 2021 Jul 12;121(8):705-714. doi: 10.1515/jom-2020-0292. J Osteopath Med. 2021. PMID: 34237804
-
Tocilizumab, an Exploratory Treatment for Severe COVID-19 Patients.Can J Infect Dis Med Microbiol. 2022 Mar 15;2022:6375870. doi: 10.1155/2022/6375870. eCollection 2022. Can J Infect Dis Med Microbiol. 2022. PMID: 35308307 Free PMC article.
-
Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy.Autoimmun Rev. 2020 Jul;19(7):102568. doi: 10.1016/j.autrev.2020.102568. Epub 2020 May 3. Autoimmun Rev. 2020. PMID: 32376398 Free PMC article. Review.
-
Tocilizumab in SARS-CoV-2 Patients with the Syndrome of Cytokine Storm: A Narrative Review.Rev Recent Clin Trials. 2021;16(2):138-145. doi: 10.2174/1574887115666200917110954. Rev Recent Clin Trials. 2021. PMID: 32940187 Review.
Cited by
-
Evaluation of Patterns of Trauma Reporting to the Emergency Department During the First COVID-19 Lockdown in India.Cureus. 2021 Apr 21;13(4):e14609. doi: 10.7759/cureus.14609. Cureus. 2021. PMID: 34040909 Free PMC article.
-
Severe acute respiratory syndrome coronavirus 2 infection: Role of interleukin-6 and the inflammatory cascade.World J Virol. 2022 May 25;11(3):113-128. doi: 10.5501/wjv.v11.i3.113. World J Virol. 2022. PMID: 35665236 Free PMC article. Review.
-
Tocilizumab in COVID-19: Is the Temptation Worthwhile?Indian J Crit Care Med. 2021 Mar;25(3):247-248. doi: 10.5005/jp-journals-10071-23750. Indian J Crit Care Med. 2021. PMID: 33790498 Free PMC article.
-
Safety profile of COVID-19 drugs in a real clinical setting.Eur J Clin Pharmacol. 2022 May;78(5):733-753. doi: 10.1007/s00228-021-03270-2. Epub 2022 Jan 28. Eur J Clin Pharmacol. 2022. PMID: 35088108 Free PMC article. Review.
References
-
- Hui DS, Azhar EI, Madani TA, Ntoumi F, Kock R, Dar O et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;;91::264––266.. doi: 10.1016/j.ijid.2020.01.009. DOI: . PMC 7128332. PMID 31953166. - DOI - PMC - PubMed
-
- Lan J, Ge JW, Yu JF, Shan SS, Zhou H, Fan SL et al. Crystal structure of the 2019-nCoV spike receptor-binding domain bound with the ACE2 receptor. https://www.biorxiv.org/content/10.1101/2020.02.19.956235v1. 2020. doi: 10.1101/2020.02.19.956235. Available from: DOI: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
