Activation of STING Signaling Pathway Effectively Blocks Human Coronavirus Infection
- PMID: 33789998
- PMCID: PMC8316077
- DOI: 10.1128/JVI.00490-21
Activation of STING Signaling Pathway Effectively Blocks Human Coronavirus Infection
Abstract
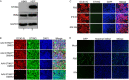
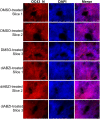
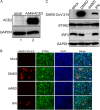
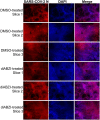
The COVID-19 pandemic poses a serious global health threat. The rapid global spread of SARS-CoV-2 highlights an urgent need to develop effective therapeutics for blocking SARS-CoV-2 infection and spread. Stimulator of Interferon Genes (STING) is a chief element in host antiviral defense pathways. In this study, we examined the impact of the STING signaling pathway on coronavirus infection using the human coronavirus OC43 (HCoV-OC43) model. We found that HCoV-OC43 infection did not stimulate the STING signaling pathway, but the activation of STING signaling effectively inhibits HCoV-OC43 infection to a much greater extent than that of type I interferons (IFNs). We also discovered that IRF3, the key STING downstream innate immune effector, is essential for this anticoronavirus activity. In addition, we found that the amidobenzimidazole (ABZI)-based human STING agonist diABZI robustly blocks the infection of not only HCoV-OC43 but also SARS-CoV-2. Therefore, our study identifies the STING signaling pathway as a potential therapeutic target that could be exploited for developing broad-spectrum antiviral therapeutics against multiple coronavirus strains in order to face the challenge of future coronavirus outbreaks.IMPORTANCE The highly infectious and lethal SARS-CoV-2 is posing an unprecedented threat to public health. Other coronaviruses are likely to jump from a nonhuman animal to humans in the future. Novel broad-spectrum antiviral therapeutics are therefore needed to control known pathogenic coronaviruses such as SARS-CoV-2 and its newly mutated variants, as well as future coronavirus outbreaks. STING signaling is a well-established host defense pathway, but its role in coronavirus infection remains unclear. In the present study, we found that activation of the STING signaling pathway robustly inhibits infection of HCoV-OC43 and SARS-CoV-2. These results identified the STING pathway as a novel target for controlling the spread of known pathogenic coronaviruses, as well as emerging coronavirus outbreaks.
Keywords: COVID-19; HCoV-OC43; SARS-CoV-2; STING; antiviral therapy; coronavirus.
Copyright © 2021 American Society for Microbiology.
Figures



Similar articles
-
Inhibition of coronavirus infection by a synthetic STING agonist in primary human airway system.Antiviral Res. 2021 Mar;187:105015. doi: 10.1016/j.antiviral.2021.105015. Epub 2021 Jan 12. Antiviral Res. 2021. PMID: 33444702 Free PMC article.
-
Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling.PLoS One. 2012;7(2):e30802. doi: 10.1371/journal.pone.0030802. Epub 2012 Feb 1. PLoS One. 2012. PMID: 22312431 Free PMC article.
-
Novel antiviral activity of PAD inhibitors against human beta-coronaviruses HCoV-OC43 and SARS-CoV-2.Antiviral Res. 2022 Apr;200:105278. doi: 10.1016/j.antiviral.2022.105278. Epub 2022 Mar 11. Antiviral Res. 2022. PMID: 35288208 Free PMC article.
-
Human Coronavirus OC43 as a Low-Risk Model to Study COVID-19.Viruses. 2023 Feb 20;15(2):578. doi: 10.3390/v15020578. Viruses. 2023. PMID: 36851792 Free PMC article. Review.
-
An overview on the seven pathogenic human coronaviruses.Rev Med Virol. 2022 Mar;32(2):e2282. doi: 10.1002/rmv.2282. Epub 2021 Aug 2. Rev Med Virol. 2022. PMID: 34339073 Review.
Cited by
-
A basally active cGAS-STING pathway limits SARS-CoV-2 replication in a subset of ACE2 positive airway cell models.Nat Commun. 2024 Sep 27;15(1):8394. doi: 10.1038/s41467-024-52803-7. Nat Commun. 2024. PMID: 39333139 Free PMC article.
-
SARS-CoV-2 Nsp15 antagonizes the cGAS-STING-mediated antiviral innate immune responses.bioRxiv [Preprint]. 2024 Sep 5:2024.09.05.611469. doi: 10.1101/2024.09.05.611469. bioRxiv. 2024. PMID: 39282446 Free PMC article. Preprint.
-
STING Agonist Induced Innate Immune Responses Drive Anti-Respiratory Virus Activity In Vitro with Limited Antiviral Efficacy In Vivo.ACS Infect Dis. 2024 Sep 13;10(9):3392-3407. doi: 10.1021/acsinfecdis.4c00504. Epub 2024 Aug 29. ACS Infect Dis. 2024. PMID: 39207884 Free PMC article.
-
An intranasal nanoparticle STING agonist protects against respiratory viruses in animal models.Nat Commun. 2024 Jul 18;15(1):6053. doi: 10.1038/s41467-024-50234-y. Nat Commun. 2024. PMID: 39025863 Free PMC article.
-
Identification of new benzofuran derivatives as STING agonists with broad-spectrum antiviral activity.Virus Res. 2024 Sep;347:199432. doi: 10.1016/j.virusres.2024.199432. Epub 2024 Jul 8. Virus Res. 2024. PMID: 38969014 Free PMC article.
References
-
- Woo PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, Bai R, Teng JL, Tsang CC, Wang M, Zheng BJ, Chan KH, Yuen KY. 2012. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol 86:3995–4008. 10.1128/JVI.06540-11. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

