Necroptosis, pyroptosis and apoptosis: an intricate game of cell death
- PMID: 33785842
- PMCID: PMC8008022
- DOI: 10.1038/s41423-020-00630-3
Necroptosis, pyroptosis and apoptosis: an intricate game of cell death
Abstract
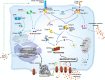
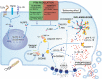
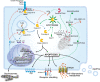
Cell death is a fundamental physiological process in all living organisms. Its roles extend from embryonic development, organ maintenance, and aging to the coordination of immune responses and autoimmunity. In recent years, our understanding of the mechanisms orchestrating cellular death and its consequences on immunity and homeostasis has increased substantially. Different modalities of what has become known as 'programmed cell death' have been described, and some key players in these processes have been identified. We have learned more about the intricacies that fine tune the activity of common players and ultimately shape the different types of cell death. These studies have highlighted the complex mechanisms tipping the balance between different cell fates. Here, we summarize the latest discoveries in the three most well understood modalities of cell death, namely, apoptosis, necroptosis, and pyroptosis, highlighting common and unique pathways and their effect on the surrounding cells and the organism as a whole.
Keywords: Apoptosis; Inflammation; Necroptosis; Pyroptosis; Survival.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Apoptosis, Pyroptosis, and Necroptosis-Oh My! The Many Ways a Cell Can Die.J Mol Biol. 2022 Feb 28;434(4):167378. doi: 10.1016/j.jmb.2021.167378. Epub 2021 Nov 25. J Mol Biol. 2022. PMID: 34838807 Review.
-
From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways.Comput Struct Biotechnol J. 2021 Aug 3;19:4641-4657. doi: 10.1016/j.csbj.2021.07.038. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34504660 Free PMC article. Review.
-
ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis).Front Cell Infect Microbiol. 2019 Nov 26;9:406. doi: 10.3389/fcimb.2019.00406. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31850239 Free PMC article. Review.
-
The Antisocial Network: Cross Talk Between Cell Death Programs in Host Defense.Annu Rev Immunol. 2021 Apr 26;39:77-101. doi: 10.1146/annurev-immunol-112019-072301. Epub 2021 Jan 13. Annu Rev Immunol. 2021. PMID: 33441019 Free PMC article.
-
Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research.J Hematol Oncol. 2022 Dec 8;15(1):174. doi: 10.1186/s13045-022-01392-3. J Hematol Oncol. 2022. PMID: 36482419 Free PMC article. Review.
Cited by
-
The pluripotent-to-totipotent state transition in mESCs activates the intrinsic apoptotic pathway through DUX-induced DNA replication stress.Cell Mol Life Sci. 2024 Oct 26;81(1):440. doi: 10.1007/s00018-024-05465-z. Cell Mol Life Sci. 2024. PMID: 39460804 Free PMC article.
-
Peimine induces apoptosis of glioblastoma cells through regulation of the PI3K/AKT signaling pathway.Exp Ther Med. 2024 Oct 3;28(6):447. doi: 10.3892/etm.2024.12737. eCollection 2024 Dec. Exp Ther Med. 2024. PMID: 39430343 Free PMC article.
-
Current insights into the regulation of programmed cell death by TP53 mutation in cancer.Front Oncol. 2022 Oct 13;12:1023427. doi: 10.3389/fonc.2022.1023427. eCollection 2022. Front Oncol. 2022. PMID: 36313700 Free PMC article. Review.
-
Saikosaponins Targeting Programmed Cell Death as Anticancer Agents: Mechanisms and Future Perspectives.Drug Des Devel Ther. 2024 Aug 21;18:3697-3714. doi: 10.2147/DDDT.S470455. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39185081 Free PMC article. Review.
-
The Function of Necroptosis and Its Treatment Target in IBD.Mediators Inflamm. 2024 Jul 31;2024:7275309. doi: 10.1155/2024/7275309. eCollection 2024. Mediators Inflamm. 2024. PMID: 39118979 Free PMC article. Review.
References
-
- Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J. Immunol. 1988;141:2629–34, 7. - PubMed
-
- Holler N, et al. Fas triggers an alternative, caspase-8–independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000;1:489–495. - PubMed
Publication types
MeSH terms
Grants and funding
- PLAT-IL-1, 714175/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- SFBTRR57/Deutsche Forschungsgemeinschaft (German Research Foundation)
- EXC 2151 - 390873048/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources
Other Literature Sources

