PUMA facilitates EMI1-promoted cytoplasmic Rad51 ubiquitination and inhibits DNA repair in stem and progenitor cells
- PMID: 33785736
- PMCID: PMC8009889
- DOI: 10.1038/s41392-021-00510-w
PUMA facilitates EMI1-promoted cytoplasmic Rad51 ubiquitination and inhibits DNA repair in stem and progenitor cells
Abstract
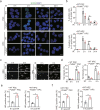
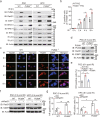
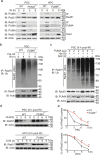
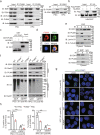
Maintenance of genetic stability via proper DNA repair in stem and progenitor cells is essential for the tissue repair and regeneration, while preventing cell transformation after damage. Loss of PUMA dramatically increases the survival of mice after exposure to a lethal dose of ionizing radiation (IR), while without promoting tumorigenesis in the long-term survivors. This finding suggests that PUMA (p53 upregulated modulator of apoptosis) may have a function other than regulates apoptosis. Here, we identify a novel role of PUMA in regulation of DNA repair in embryonic or induced pluripotent stem cells (PSCs) and immortalized hematopoietic progenitor cells (HPCs) after IR. We found that PUMA-deficient PSCs and HPCs exhibited a significant higher double-strand break (DSB) DNA repair activity via Rad51-mediated homologous recombination (HR). This is because PUMA can be associated with early mitotic inhibitor 1 (EMI1) and Rad51 in the cytoplasm to facilitate EMI1-mediated cytoplasmic Rad51 ubiquitination and degradation, thereby inhibiting Rad51 nuclear translocation and HR DNA repair. Our data demonstrate that PUMA acts as a repressor for DSB DNA repair and thus offers a new rationale for therapeutic targeting of PUMA in regenerative cells in the context of DNA damage.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
DNA repair in primordial follicle oocytes following cisplatin treatment.J Assist Reprod Genet. 2021 Jun;38(6):1405-1417. doi: 10.1007/s10815-021-02184-3. Epub 2021 Apr 16. J Assist Reprod Genet. 2021. PMID: 33864208 Free PMC article.
-
Phosphorylation of Ago2 is required for its role in DNA double-strand break repair.J Genet Genomics. 2021 Apr 20;48(4):333-340. doi: 10.1016/j.jgg.2021.03.011. Epub 2021 Apr 24. J Genet Genomics. 2021. PMID: 34039517
-
Akt1 Stimulates Homologous Recombination Repair of DNA Double-Strand Breaks in a Rad51-Dependent Manner.Int J Mol Sci. 2017 Nov 20;18(11):2473. doi: 10.3390/ijms18112473. Int J Mol Sci. 2017. PMID: 29156644 Free PMC article.
-
Genetic variations in DNA repair genes, radiosensitivity to cancer and susceptibility to acute tissue reactions in radiotherapy-treated cancer patients.Acta Oncol. 2008;47(5):809-24. doi: 10.1080/02841860801885969. Acta Oncol. 2008. PMID: 18568480 Review.
-
DNA repair in murine embryonic stem cells and differentiated cells.Exp Cell Res. 2008 Jun 10;314(9):1929-36. doi: 10.1016/j.yexcr.2008.02.007. Epub 2008 Feb 26. Exp Cell Res. 2008. PMID: 18374918 Free PMC article. Review.
Cited by
-
Overabundance of Veillonella parvula promotes intestinal inflammation by activating macrophages via LPS-TLR4 pathway.Cell Death Discov. 2022 May 6;8(1):251. doi: 10.1038/s41420-022-01015-3. Cell Death Discov. 2022. PMID: 35523778 Free PMC article.
-
KAT6A Condensates Impair PARP1 Trapping of PARP Inhibitors in Ovarian Cancer.Adv Sci (Weinh). 2024 Sep;11(34):e2400140. doi: 10.1002/advs.202400140. Epub 2024 Jul 8. Adv Sci (Weinh). 2024. PMID: 38973255 Free PMC article.
-
Effect of Emi1 gene silencing on the proliferation and invasion of human breast cancer cells.BMC Mol Cell Biol. 2023 Dec 1;24(1):34. doi: 10.1186/s12860-023-00494-1. BMC Mol Cell Biol. 2023. PMID: 38041032 Free PMC article.
-
DNA Damage Response and Cell Cycle Regulation in Pluripotent Stem Cells.Genes (Basel). 2021 Sep 29;12(10):1548. doi: 10.3390/genes12101548. Genes (Basel). 2021. PMID: 34680943 Free PMC article. Review.
-
Roles of USP9X in cellular functions and tumorigenesis (Review).Oncol Lett. 2023 Oct 10;26(6):506. doi: 10.3892/ol.2023.14093. eCollection 2023 Dec. Oncol Lett. 2023. PMID: 37920433 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

